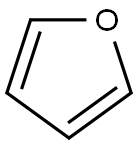
푸란
|
|
푸란 속성
- 녹는점
- -85.6 °C
- 끓는 점
- 67 °C10 mm Hg(lit.)
- 밀도
- 0.936 g/mL at 25 °C(lit.)
- 증기 밀도
- 2.35 (vs air)
- 증기압
- 1672 mm Hg ( 55 °C)
- 굴절률
- n
20/D 1.5070(lit.)
- 인화점
- 160 °F
- 저장 조건
- Store below +30°C.
- 용해도
- 알코올: 자유롭게 용해됨
- 물리적 상태
- 액체
- 색상
- 무색~황색으로 투명함
- 냄새
- 0.10?%?in?프로필렌 글리콜에서. 미묘한
- Odor Threshold
- 9.9ppm
- 폭발한계
- 2.3-14.3%(V)
- 수용성
- 불용성
- 감도
- Air & Light Sensitive
- Merck
- 14,4296
- BRN
- 103221
- Dielectric constant
- 3.0(25℃)
- 노출 한도
- NIOSH: IDLH 13 ppm
- 안정성
- 안정적인. 피해야 할 물질에는 강한 산화제, 산, 과산화물 및 산소가 포함됩니다. 가연성이 높습니다. 공기와 폭발성 혼합물을 형성할 수 있음.
- InChIKey
- YLQBMQCUIZJEEH-UHFFFAOYSA-N
- LogP
- 1.34
- CAS 데이터베이스
- 110-00-9(CAS DataBase Reference)
- IARC
- 2B (Vol. 63) 1995
- NIST
- Furan(110-00-9)
- EPA
- Furan (110-00-9)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | T,F+ | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 45-12-19-20/22-38-48/22-52/53-68 | ||
| 안전지침서 | 53-45-61- | ||
| 유엔번호(UN No.) | UN 2811 6.1/PG 2 | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | OB3870000 | ||
| F 고인화성물질 | 8-9-23 | ||
| 자연 발화 온도 | 390 °C | ||
| TSCA | Yes | ||
| HS 번호 | 2932 19 00 | ||
| 위험 등급 | 3 | ||
| 포장분류 | I | ||
| 유해 물질 데이터 | 110-00-9(Hazardous Substances Data) | ||
| 독성 | LC (in air) in rats: 30400 ppm (Henderson) | ||
| IDLA | 13 ppm (35.1 mg/m3) | ||
| 기존화학 물질 | KE-17309 | ||
| 중점관리물질 필터링 | 별표2-33 |
푸란 C화학적 특성, 용도, 생산
개요
Furan occurs naturally in oils distilled from rosin containing pinewood. In addition, many natural foods contain the furan ring structure and substituted furans may be formed through cooking of simple carbohydrates. Furan is also found in tobacco smoke as well as wood smoke and gas emissions from gasoline and diesel engines. Furan has also been detected in industrial effluents and can be emitted to the air from petroleum refineries and coal-mining and gasification plants.화학적 성질
Furan is a cyclic dienic ether stabilized by benzene-like resonance. Because of its conjugated unsaturation and heterocyclic atom, furan will undergo many types of reactions. It is, therefore, of interest as a chemical intermediate for pharmaceuticals, insecticides and fine chemicals. The heterocyclic oxygen atom in a ring with conjugated unsaturation gives furan a combination of ether, aromatic and olefinic characteristics. This polyfunctionality permits it to undergo a variety of reactions. Compared to benzene, the furan ring has greater reactivity, and is more susceptible to cleavage, thus resembling the vinyl ethers. Like the vinyl ethers, the furan ring is cleaved by aqueous acids. This reaction is accompanied by resinification .용도
Furan is a five-membered heterocyclic aromatic ring. Furan is used as a building block for the preparation of many heterocyclic compounds.정의
A heterocyclic liquid organic compound. Its five-membered ring contains four carbon atoms and one oxygen atom. The structure is characteristic of some monosaccharide sugars (furanoses).일반 설명
Furan is a stable, colourless chemical liquid. It is highly flammable and forms explosive mixtures with air. It is incompatible with many other chemical substances, for example, strong oxidising agents, acids, peroxides, and oxygen. Furan is produced commercially by decarbonylation of furfural. It is used mainly in the production of tetrahydrofuran, thiophene, and pyrrole. It also occurs naturally in certain woods and during the combustion of coal and is found in engine exhausts, wood smoke, and tobacco smoke.공기와 물의 반응
Highly flammable. When uninhibited, Furan forms explosive peroxides on exposure to air. Insoluble in water.반응 프로필
Furan is sensitive to heat and may turn brown upon standing. Furan may be light sensitive. When uninhibited, Furan forms explosive peroxides on exposure to air. Furan may react with oxidizers, acids, peroxides and oxygen. Furan resinifies on evaporation or when in contact with mineral acids, but Furan is stable in alkalis. .위험도
Flammable, dangerous fire risk, flammable limits 2–24%, forms peroxides on exposure to air. Absorbed by skin. Possible carcinogen.건강위험
The vapors are narcotic. Acute exposure to Furan by inhalation may involve both reversible and irreversible changes. Acute exposure by ingestion or skin absorption, as well as chronic exposure, are associated with high toxicity.화재위험
Very dangerous, upon exposure to heat or flame. Furan may form unstable peroxides on exposure to air. Contact with acids can initiate a violent, heat producing reaction. Avoid acids, oxidizing agents. Upon standing in air, Furan may form unstable peroxides.Safety Profile
Confirmed carcinogen. Poison by inhalation and intraperitoneal routes. Moderately toxic by ingestion and skin contact. Experimental reproductive effects. A narcotic. Mutation data reported. The exposure concentration limit of 10 ppm together with its low boiling point requires that adequate ventilation be provided in areas where ths chemical is handled. Contact with liquid must be avoided since this chemical can be absorbed through the skin. Washing thoroughly with soap and water followed by prolonged rinsing should be done immedlatelp after accidental contact. A very dangerous fire hazard when exposed to heat or flame; can react with oxidzing materials. Unstabdized, it may form unstable peroxides on exposure to air and should always be tested before ddlation. Washing with an aqueous solution of ferrous sulfate slightly acidified with sodum bisulfate will remove these peroxides. Confirm by test. Contact with acids can initiate a violent exothermic reaction. Moderate explosion hazard when exposed to flame. Furan's low bohg point makes it easy to obtain explosive concentrations of the vapor in inadequately ventilated areas. To fight fire, use CO2, dr). chemical. When heated to decomposition it emits acrid smoke and irritating fumes. See also PEROXIDES.잠재적 노출
Furan is used as a chemical intermedi ate in the production of herbicides and pharmaceuticals; for making tetrahydrofuran; in formation of lacquers; as a sol vent for resins in organic synthesis, especially for pyrrole, thiophene.Carcinogenicity
Furan is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals.환경귀착
Furan may be released to the environment as a waste industrial product or from unintentional or accidental releases. If released to soil, it is expected to volatilize. If released to water, furan is not expected to adsorb to suspended particles and sediment and is likely to volatilize to ambient air. Sulfate-reducing bacteria can degrade furan. However, under nonsulfate-reducing conditions, biodegradation in soil and water is expected to be slow. In the air, furan will exist as a vapor and will be subject to degradation by reacting with hydroxyl radicals.운송 방법
UN2389 Furan, Hazard Class: 3; Labels: 3-Flammable liquid.Purification Methods
Shake it with aqueous 5% KOH, dry it with CaSO4 or Na2SO4, then distil it under nitrogen, from KOH or sodium, immediately before use. A trace of hydroquinone could be added as an inhibitor of oxidation. [Beilstein 17 H 27, 17 I 16, 17 II 34, 17/1 V 291.]비 호환성
May form explosive mixture with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong acids, amines, strong bases, reducing agents. Unless stabilized with an inhibitor, air exposure forms unstable peroxides.폐기물 처리
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations govern ing storage, transportation, treatment, and waste disposal.푸란 준비 용품 및 원자재
원자재
준비 용품
Fluvastatin sodium salt
Cefetamet hydrochloride
Cabergoline
비스피리박-소듐
2-NITROFURAN
EXO-3,6-EPOXY-1,2,3,6-TETRAHYDROPHTHALIC ANHYDRIDE
2아세틸퓨란
Suprofen
2-[3-(2-FURYL)PHENYL]-4,4,5,5-TETRAMETHYL-1,3,2-DIOXABOROLANE
피롤
클라드리빈
메실산가벡세이트
카보퓨란
1,4-EPOXY-1,4-DIHYDRONAPHTHALENE
페노티오캅
2,5-Dimethoxytetrahydrofuran
3-CHLORO-2-METHYLBIPHENYL
에파비렌즈
Cefuroxime
polypyrrole-polyvinyl chloride composite film
DELAVIRDINE
2-HEXANOYLFURAN
2-Bromofuran
2-PROPIONYLFURAN
푸란 공급 업체
글로벌( 227)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| Jiangsu Qingquan Chemical Co., Ltd. | +86-571-86589381,86589382,86589383 |
sales1@qqpharm.com | CHINA | 154 | 55 |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 |
sales@sdzschem.com | China | 2931 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 |
sales@conier.com | China | 49390 | 58 |
| SIMAGCHEM CORP | +86-13806087780 |
sale@simagchem.com | China | 17367 | 58 |
| Career Henan Chemica Co | +86-0371-86658258 15093356674; |
laboratory@coreychem.com | China | 30255 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 |
sales@tnjchem.com | China | 34572 | 58 |
| ANHUI WITOP BIOTECH CO., LTD | +8615255079626 |
eric@witopchemical.com | China | 23556 | 58 |
| AFINE CHEMICALS LIMITED | 0571-85134551 |
info@afinechem.com | CHINA | 15377 | 58 |
푸란 관련 검색:
1,3-부타디엔 폴리(에피클로로히드린) 테트라하이드로푸란 푸르푸랄 2,3,7,8-테트라클로로디벤조-P-디옥신 무수프탈산 티오펜 메틸(5-)-2-퍼퓨랄 카보퓨란 벤조퓨란
Carbamic acid, 3-furanyl-, 1,1-dimethylethyl ester (9CI)
3-FURANECARBOXAMIDE
3-Furanboronic acid pinacol ester
4-Nitrobenzonitrile
Faropenem sodium
Lafutidine
METHYL FUROATE
(2-METHYL-PYRIDIN-3-YL)-METHANOL