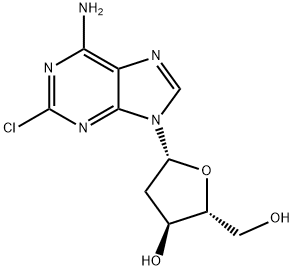
클라드리빈
|
|
클라드리빈 속성
- 녹는점
- 181-185 °C(lit.)
- 알파
- D25 -18.8° (c = 1 in DMF)
- 끓는 점
- 547.6±60.0 °C(Predicted)
- 밀도
- 2.03±0.1 g/cm3(Predicted)
- 저장 조건
- -20°C
- 용해도
- 물에 약간 용해되고, 디메틸 설폭사이드에 용해되고, 메탄올에 약간 용해되고, 아세토니트릴에는 거의 용해되지 않습니다. 다형성(5.9)을 보여줍니다.
- 산도 계수 (pKa)
- 13.75±0.60(Predicted)
- 물리적 상태
- 흰색 고체
- 색상
- 흰색에서 연한 노란색
- 최대 파장(λmax)
- 265nm(EtOH aq.)(lit.)
- Merck
- 14,2337
- 안정성
- 냉동고에 보관
- InChIKey
- PTOAARAWEBMLNO-HSUXUTPPSA-N
- CAS 데이터베이스
- 4291-63-8(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | T | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 36/37/38-23/24/25 | ||
| 안전지침서 | 26-37/39-45-36-22 | ||
| 유엔번호(UN No.) | UN 2811 6.1 / PGIII | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | AU7357560 | ||
| HS 번호 | 29349990 | ||
| 유해 물질 데이터 | 4291-63-8(Hazardous Substances Data) | ||
| 독성 | LD50 intraperitoneal in mouse: 150mg/kg |
클라드리빈 C화학적 특성, 용도, 생산
개요
Cladribine, an adenosine deaminase inhibitor, was introduced in the United States as a single intravenous treatment for hairy cell leukemia. The incorporation of a chlorine atom at the 2-position of deoxyadenosine renders cladribine more resistant to enzymatic attack by adenosine deaminase, resulting in a more prolonged cytotoxic effect. Cladribine efficiently crosses lymphocyte and monocyte cell membranes and is metabolized in cells to the biologically active triphosphate, which inhibits DNA synthesis. While most antineoplastic drugs are active primarily against dividing cells, cladribine destroys both resting and proliferating cells. Its potential uses in the treatment of autoimmune hemolytic anemia, multiple sclerosis, chronic lymphocytic leukemia and various lymphomas have also been evaluated.화학적 성질
White Crystalline Solid용도
It is a substituted purine nucleoside with antileukemic activityIndications
Cladribine (Leustatin) is a synthetic purine nucleoside that is converted to an active cytotoxic metabolite by the enzyme deoxycytidine kinase. Like the other purine antimetabolites, it is relatively selective for both normal and malignant lymphoid cells and kills resting as well as dividing cells by mechanisms that are not completely understood.The drug is highly active against hairy cell leukemia, producing complete remissions in more than 60% of patients treated with a single 7-day course. Activity has also been noted in other low-grade lymphoid malignancies. The major side effect is myelosuppression.
정의
ChEBI: 2'-Deoxyadenosine in which the hydrogen at position 2 on the purine ring has been substituted by chlorine. It inhibits the synthesis and repair of DNA, particularly in lymphocytes and monocytes, and is used as an antimetabolite antineoplastic drug for the treatment of lymphoid malignancies including hairy-cell leukaemia and chronic lymphocytic leukaemia.일반 설명
The drug is available in a 10-mg or 10-mL single-use vialfor IV use. Cladribine is used for chronic lymphocyticleukemia, hairy cell leukemia, and non-Hodgkin’s lymphoma.The mechanism of action of this purine deoxyadenosineanalog involves incorporation into DNA resultingin inhibition of DNA chain extension and inhibitionof DNA synthesis and function. This incorporation intoDNA occurs via the triphosphate metabolite active species.The 2-chloro group on the adenine ring produces resistanceto breakdown by adenosine deaminase. Resistance to the anticancereffects can occur because of decreased expressionof the activating enzyme or overexpression of the catabolicenzymes. Oral bioavailability is variable and averages about50%. The drug crosses the blood-brain barrier; however,CSF concentrations reach only 25% of those in plasma. Thedrug is selectively activated inside the cell, and intracellularconcentrations of phosphorylated metabolites exceed thosein plasma. Toxicities include myelosuppression, neutropenia,immunosuppression, fever, nausea, and vomiting.클라드리빈 준비 용품 및 원자재
원자재
Sodium hydride 50%
anhydrous Acetonitrile
실리카겔
2,6-Dichloropurine
클로로포름
푸란
4-다이메틸아미노피리딘
트라이-엔-뷰틸주석 하이드라이드
[1,2-Bis(diphenylphosphino)ethane]dichloropalladium(II)
염화나트륨
아조비스이소부티로니트릴
1,1,3,3-TETRAISOPROPYLDISILOXANE
준비 용품
클라드리빈 공급 업체
글로벌( 474)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 |
sales@hbmojin.com | China | 12455 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 |
info@fdachem.com | China | 16803 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +8618740459177 |
sarah@tnjone.com | China | 964 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 |
sales@capotchem.com | China | 29797 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21689 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 |
fandachem@gmail.com | China | 9344 | 55 |
| career henan chemical co | +86-0371-86658258 |
sales@coreychem.com | China | 29914 | 58 |
| Hainan Xiyuan Chemical Technology Co., Ltd. | +86-15008981366 |
15008981366@163.com | CHINA | 9 | 58 |
| Biochempartner | 0086-13720134139 |
candy@biochempartner.com | CHINA | 967 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |
클라드리빈 관련 검색:
아데노신 삼인산 Na염 3′,5′-사이클릭아데닐산 아데노신 트라이포스파타아제 크리센 클로로디플루오르메탄 클라드리빈
2'-Deoxyadenosine monohydrate
EC 3.5.4.4
5'-Deoxyadenosylcobalamin
Vidarabine
S-ADENOSYL-L-METHIONINE
2'-Deoxyadenosine
Clofarabine
2-Chloroadenosine
2-Chloroadenosine hemidydrate
DNA, SINGLE STRANDED, IMM. ON CELLULOSE, FROM CALF THYMUS*
Cladribine Related Compound A
2-CHLOROADENOSINE-3',5'-CYCLIC MONOPHOSPHATE SODIUM SALT