Caffeine suppliers
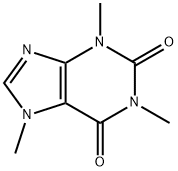
Caffeine
- CAS:
- 58-08-2
- MF:
- C8H10N4O2
- MW:
- 194.19
Properties
- Melting point:
- 234-236.5 °C(lit.)
- Boiling point:
- 178°C
- Density
- 1.23
- refractive index
- 1.6590 (estimate)
- FEMA
- 2224 | CAFFEINE
- Flash point:
- 178°C
- storage temp.
- 2-8°C
- solubility
- Sparingly soluble in water, freely soluble in boiling water, slightly soluble in ethanol (96 per cent). It dissolves in concentrated solutions of alkali benzoates or salicylates.
- pka
- pKa 0.6 (Uncertain)
- form
- Crystals or Crystalline Powder
- color
- Silky white or white
- PH
- pH (10g/l, 25℃) : 5.5~6.5
- Odor
- at 100.00 %. odorless
- Odor Type
- odorless
- Water Solubility
- 20 g/L (20 ºC)
- Sublimation
- 178 ºC
- Merck
- 14,1636
- BRN
- 17705
- Stability:
- Stable. Incompatible with strong acids, strong bases, strong oxidizing agents, iodine, silver salts, tannins. Weakly light sensitive in solution.
- LogP
- -0.07
Safety Information
- Symbol(GHS)

GHS07
- Signal word
- Warning
- Hazard statements
- H302
- Precautionary statements
- P301+P312+P330
- Hazard Codes
- Xn,T,F
- Risk Statements
- 22-25-39/23/24/25-23/24/25-11
- Safety Statements
- 16-36/37-45-7
- RIDADR
- UN 1544 6.1/PG 3
- WGK Germany
- 1
- RTECS
- EV6475000
- F
- 10
- TSCA
- Yes
- HazardClass
- 6.1
- PackingGroup
- III
- HS Code
- 29393000
- Toxicity
- LD50 orally in mice, hamsters, rats, rabbits (mg/kg): 127, 230, 355, 246 (males); 137, 249, 247, 224 (females) (Palm)
Use
Caffeine may be hygroscopic. Aqueous solutions (1.12 mg/mL) are stable for three weeks at 41° F if protected from light. In normal room lighting and at room temperature, solutions are stable for 3 days. Solutions of Caffeine in water, DMSO, 95% ethanol or acetone should be stable for 24 hours under normal lab conditions. REACTIVITY: Caffeine may react with strong oxidizing agents. Caffeine is also incompatible with iodine, silver salts and tannins. Caffeine is a very weak base. Caffeine is decomposed by strong solutions of caustic alkalis.
According to relevant laws, regulations, policies and the provisions of this website, this website does not display the product information!
The source is "易制毒化学品". If the information is inaccurate, please contact the platform to modify it.
contact information:info@chemicalbook.com 400-158-660