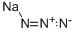Sodium azide suppliers
Sodium azide
- CAS:
- 26628-22-8
- MF:
- N3Na
- MW:
- 65.01
Properties
- Melting point:
- 275 °C
- Boiling point:
- 300 °C
- Density
- 1.85
- vapor pressure
- 0Pa at 20℃
- Flash point:
- 300 °C
- storage temp.
- 2-8°C
- solubility
- H2O: 1 M at 20 °C, clear, colorless
- form
- Powder/Solid
- pka
- pK = 4.8, aq solns contains HN3 which escapes readily at 37°
- color
- White to off-white
- Specific Gravity
- 1.85
- Odor
- Odorless solid
- Water Solubility
- 420 g/L (17 ºC)
- Sensitive
- Air & Moisture Sensitive
- Merck
- 14,8581
- Exposure limits
- Ceiling 0.3 mg/m3 in air (ACGIH).
- Stability:
- Unstable. Avoid heat, sources of ignition, moisture, shock, friction. Incompatible with strong oxidizing agents, mineral acids, water, halogen acids and halogen compounds, barium carbonate, bromine, carbon disulphide, mercury, dimethyl sulphate, common metals, especially brass, copper, lead, silver, strong acids.
Safety Information
- Symbol(GHS)



GHS06,GHS08,GHS09
- Signal word
- Danger
- Hazard statements
- H300+H310+H330-H373-H410
- Precautionary statements
- P262-P273-P280-P302+P352+P310-P304+P340+P310-P314
- Hazard Codes
- Xn,T,N,T+
- Risk Statements
- 28-32-50/53-52/53-22-27-21/22
- Safety Statements
- 61-60-45-28A-28-36/37
- RIDADR
- UN 1687 6.1/PG 2
- WGK Germany
- 2
- RTECS
- VY8050000
- TSCA
- Yes
- HazardClass
- 6.1
- PackingGroup
- II
- HS Code
- 28500060
- Toxicity
- LD50 in rats (mg/kg): 45 orally (Frederick, Babish)
Use
Biomedical Sciences Sodium azide is a biocide that is used in making chemical preservatives that are used in laboratories and hospitals. In hospitals, it is used as a preservative in blood tests and diagnostic machines. Since the chemical is soluble, it can act as useful metabolic inhibitors that can affect the activities of numerous oxidative enzymes. Specifically, it inhibits oxidative enzymes that are involved in electron transport system respiration. However, accidents have occurred whenever sodium azide is used in a hospital setting; therefore, major bodies such as the CDC that deal with chemicals do not advise the use of the compound. Automobiles Airbags Before the 1990s, sodium azide was used in airbags. In airbags, when electricity heats sodium azide to approximately 300oC, it decomposes into two simple components, namely sodium and nitrogen gas. The decomposition of sodium azide happens very fast that the filling of the airbag with nitrogen gas occurs at a velocity of around 200 miles per hour. Agriculture Sodium azide is used for pest control due to its ready solubility in water. Nitrifying bacteria acts as a catalyst that decomposes the sodium azide solution to release nitrate, HN3, and NH4+ when added to the soil. It is noteworthy that these components have wide range of activity against nematodes, weeds, and phytopathogenic fungi that is soil-borne. The chemical is typically applied through drip irrigation to avoid reactivity of HN3 in the soil-air space that can eventually result in an active compound that can be ineffective for pest control. Detonators When combined with other metals such as copper (Cu), lead (Pb), and mercury (Hg), sodium azide becomes unstable and explosive. As such, it is mostly used as detonators due to its explosive nature. A summary of main uses is listed in the table below: Industry Application Role/benefit Chemical manufacture Preparation of other inorganic azide compounds, such as lead azide and silver azide Precursor Preparation of organic azide compounds Azidation agent Agriculture Pest control of soil-borne pathogens Biocide Crop selection of plants such as rice,barley or oats Mutagen Pharmaceutical Preparation of tetrazoles pharmaceutical intermediates Raw material Airbags Automobile airbags and aircraft safety chutes Gas source/generates n2 after automobile crash Antisepsis and sterilization Preservation of bulk reagents and stock solutions in hospitals and laboratories Biocide/inhibits the cytochrome oxidase in gram-negative bacteria Photographic emulsion Preservative/works without influencing the performance of photographic emulsion Explosive Manufacture of detonating agent(lead azide) Raw material Others Manufacture of synthetic resin Foaming agent/generates N2 during the reaction Manufacture of Na Raw material/2NaN32Na+3N2
According to relevant laws, regulations, policies and the provisions of this website, this website does not display the product information!
The source is "易制爆、炸药、化学武器等". If the information is inaccurate, please contact the platform to modify it.
contact information:info@chemicalbook.com 400-158-660