- Metacyclin
-

- $0.00 / 1Kg
-
2020-05-25
- CAS:914-00-1
- Min. Order: 1KG
- Purity: 99.0%+
- Supply Ability: 300 MT
|
| | METHACYCLINE Basic information |
| Product Name: | METHACYCLINE | | Synonyms: | methacyclinebase;tri-methacycline;(4S,4aR,5S,5aR,12aS)-4-(Dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,5,10,12,12a-pentahydroxy-6-methylene-1,11-dioxo-2-naphthacenecarboxamide;6-Methyleneoxytetracycline, Metacycline, RondoMycin;METHACYCLINE;,10,12,12a-pentahydroxy-6-methylene-1,11-dioxo-;10,12,12a-pentahydroxy-6-methylene-1,11-dioxo-,(4s-(4alpha,4aalpha,5alpha,5aal;12aalpha))-ph | | CAS: | 914-00-1 | | MF: | C22H22N2O8 | | MW: | 442.42 | | EINECS: | 213-017-5 | | Product Categories: | | | Mol File: | 914-00-1.mol | 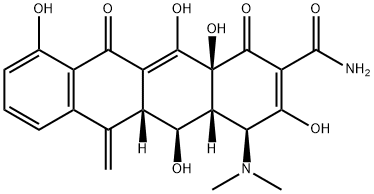 |
| | METHACYCLINE Chemical Properties |
| Melting point | 205 °C (decomp) | | Boiling point | 553.06°C (rough estimate) | | density | 1.4033 (rough estimate) | | refractive index | 1.6500 (estimate) | | storage temp. | Refrigerator | | solubility | Methanol (Slightly), Water (Slightly) | | form | Solid | | pka | pKa 3.5 (Uncertain);7.6 (Uncertain);9.5(EtOH/H2O
t=20.0) (Uncertain) | | color | Yellow to Brown | | Water Solubility | 7.548g/L(21 ºC) |
| | METHACYCLINE Usage And Synthesis |
| Description | Methacycline was synthesized by Pfizer Research Laboratories in 1961 starting with oxytetracycline. It shows two to fivefold greater in vitro antibacterial activity and in vivo protective activity than tetracycline. Methacycline has been given orally to treat infections by Rickettsia, Chlamydiae, Staphyloccus, Streptococcus, Neisseria, Klebsiella, Proteus, Escherichia coli, and Haemophilus influenzae. | | Originator | Rondomycin ,Pfizer ,UK ,1963 | | Uses | Methacycline is a tetracycline with broad range antibacterial and antiprotozoal properties. Potential veterinary drug. | | Uses | Methacycline is used for the same indications as other antibiotics of the tetracycline series.
In some cases it is tolerated better than tetracyclines. Synonyms of this drug are ron�domycin, methamycin, adramycin, and others. | | Uses | Methacycline is a semi-synthetic tetracycline prepared by dehydration of the 6-hydroxy group of oxytetracycline to yield an exocyclic 6-methylene. Like all tetracyclines, methacycline shows broad spectrum antibacterial and antiprotozoan activity and acts by binding to the 30S and 50S ribosomal subunits, blocking protein synthesis. Methacycline has been extensively cited in the literature with over 400 references. | | Definition | ChEBI: A tetracycline that is the 6-methylene analogue of oxytetracycline, obtained by formal dehydration at position 6. | | Manufacturing Process | To a stirred solution of 4.6 g (0.01 mol) of anhydrous oxytetracycline in 40 ml of dry tetrahydrofuran is added 3.5 g (0.021 mol) of pyridine-sulfur trioxide complex. After 16 hours of stirring at room temperature, the resulting suspension is filtered, and the solid is slurried with 25 ml of 2% hydrochloric acid for 10 minutes, filtered and thoroughly washed with methanol followed by ether. The pale yellow crystalline 5-oxytetracycline-6,12-hemiketal-12-sulfuric acid ester melts at 210°C.
500 mg 5-oxytetracycline-6,12-hemiketal-12-sulfuric acid ester, prepared as
described, is added to 4 ml dry liquid hydrogen fluoride, and the mixture is
stirred for 1.5 hours at ice bath temperature. The hydrogen fluoride is then
evaporated in a stream of nitrogen and the resulting gummy solids are
triturated with about 15 ml ether and filtered. The resulting solid hydrofluoride
salt is further purified by suspending in water, adjusting the pH to about 4,
and extracting the 6-methylene-5-oxytetracycline free base from the aqueous
phase with ethyl acetate. The extract is separated and evaporated to dryness
under reduced pressure. The resulting residue is triturated with ether and
filtered, and the solid is recrystallized from methanol-acetone-etherconcentrated hydrochloric acid to obtain the product as a purified
hydrochloride, according to US Patent 3,026,354. | | Brand name | Rondomycin (Medpointe). | | Therapeutic Function | Antibiotic | | Pharmaceutical Applications | 6-Methylene-5-hydroxy-tetracycline. A semisynthetic derivative
supplied as the hydrochloride for oral administration.
Mean peak plasma concentrations of 2–6 mg/L are found
about 4 h after a 300 mg oral dose. Both food and milk reduce
uptake by half. Protein binding is 80–90%. The plasma elimination
half-life varies between 7 and 15 h and increases to 44 h
in severe renal impairment. It is widely distributed, producing
lung concentrations similar to, or greater than, the simultaneous
plasma concentration. About one-third is excreted in the urine.
Gastrointestinal intolerance is reported to be less frequent
than with other tetracyclines, largely because of the lower dosages
used. There are no unique adverse drug reactions,
although skin and conjunctival pigmentation have been
reported. | | Synthesis | Methacycline, 4-dimethylamino-1,4,4a,5,5a,6,11,12a-octahydro-3,6,10,
12,12a-pentahydroxy-6-methylen-1,11-dioxo-2-naphthacencarboxamide (32.3.6), is synthe�sized from oxytetracycline (32.3.2), which is reacted with a sulfur trioxide—pyridine
complex, resulting in an oxidation reaction. Simultaneous sulfonation gives a
naphthacen–sulfotetrahydrofuran derivative intermediate (32.3.5), which when reacted with
hydrofluoric acid forms methacycline (32.3.6). |
| | METHACYCLINE Preparation Products And Raw materials |
|