|
ChemicalBook Optimization Suppliers |
|
| 化学名: | 補酵素Q10 | | 英語化学名: | Coenzyme Q10 | | 别名: | UBIQUINONE Q10;,34,38-tetracontadecaenyl)-5,6-dimethoxy-3-methyl-;2,5-cyclohexadiene-1,4-dione,2-(3,7,11,15,19,23,27,31,35,39-decamethyl-2,6,10,;22,26,30,34,38-tetracontadecaenyl)-5,6-dimethoxy-3-methyl-1(all-e)-1;5-Cyclohexadiene-1,4-dione,2-(3,7,11,15,19,23,27,31,35,39-decamethyl-2,6,10,14,18,22,26,30,34,38-t2;coenzymeq(sub10);coq(sub10);neuquinon | | CAS番号: | 303-98-0 | | 分子式: | C59H90O4 | | 分子量: | 863.34 | | EINECS: | 206-147-9 | | カテゴリ情報: | chemical reagent;pharmaceutical intermediate;phytochemical;reference standards from Chinese medicinal herbs (TCM).;standardized herbal extract;Vitamin series;Drug bulk;Mixed Fatty Acids;Antioxidant;Benzoquinones;Biochemistry;Vitamin Related Compounds;Vitamins;Nutritional Supplements;Drugs & Medication;Fatty Acid Derivatives & Lipids;Glycerols;Inhibitors;Steroids;BENUTRI;303-98-0 | | Mol File: | 303-98-0.mol | 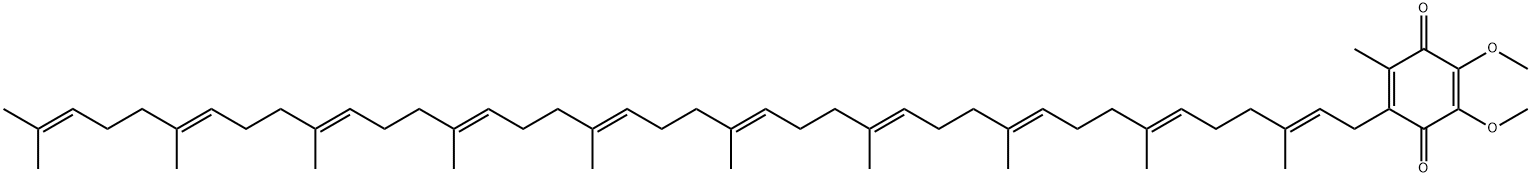 |
| 融点 | 49-51 °C | | 沸点 | 715.32°C (rough estimate) | | 比重(密度) | 0.9145 (rough estimate) | | 屈折率 | 1.4760 (estimate) | | 貯蔵温度 | -20°C | | 溶解性 | Soluble in chloroform. | | 色 | Light Orange to Dark Orange | | Sensitive | Light Sensitive | | Merck | 14,9843 | | BRN | 1900141 | | 安定性: | Stable, but may be light or heat sensitive. Store in the dark at -20 C. Incompatible with strong oxidizing agents. | | InChIKey | ACTIUHUUMQJHFO-DQXDOXBUSA-N | | LogP | 19.119 (est) | | CAS データベース | 303-98-0(CAS DataBase Reference) | | EPAの化学物質情報 | Ubidecarenone (303-98-0) |
| 主な危険性 | Xi | | Rフレーズ | 36/37/38 | | Sフレーズ | 22-24/25-26 | | WGK Germany | 3 | | RTECS 番号 | DK3900000 | | F | 8-10 | | TSCA | Yes | | HSコード | 29146990 | | 毒性 | LD50 intramuscular in mouse: > 500mg/kg |
| | 補酵素Q10 Usage And Synthesis |
| 外観 | 黄色〜黄赤色, 粉末又は塊 | | 定義 | 本品は、次の化学式で表される芳香族ケトンである。 | | 溶解性 | 多くの有機溶媒に易溶。ベンゼンに易溶、エタノールに難溶、水に不溶。水,メタノール,エタノールにほとんど不溶、クロロホルム,ヘキサン,エーテルに易溶、アセトンにやや可溶。 | | 解説 | 補酵素Q10,抗酸化作用のある脂溶性のビタミン様物質、ユビキノンの別称。同名はサプリメント剤や化粧品でよく使用される。 小学館 デジタル大辞泉について 情報 | 凡例 | | 用途 | 薬理研究用。 | | 用途 | HPLCによるユビキノン10定量用標準品。 | | 用途 | 細胞内ミトコンドリアのエネ ルギー補酵素として作用します。 | | 用途 | 細胞内ミトコンドリアのエネ
ルギー補酵素として作用します。 | | 化粧品の成分用途 | 保湿.湿潤剤、皮膚コンディショニング剤、皮膚保護剤、酸化防止剤 | | 効能 | 強心薬, 補酵素, ATP産生賦活薬 | | 製法 | 食品では魚介類や肉類に多く含まれるが、体内でも生合成され、ベンゾキノン部がフェニルアラニン、イソプレン部がアセチルCoAから合成される。 | | 商品名 | ノイキノン (エーザイ); ユビデカレノン (日新製薬-山形) | | 説明 | Coenzyme Q10 (CoQ10) is a quinone that is found throughout the human body in cell membranes, primarily in mitochondrial membranes, with the highest levels in the heart, lungs, liver, kidneys, spleen, pancreas, and adrenal glands. It exists in three redox states: fully oxidized (CoQ10/ubiquinone), partially reduced (semiquinone or ubisemiquinone), and fully reduced (ubiquinol; ). CoQ10 acts as an electron shuttle in the electron transport chain via its reduction to ubiquinol between mitochondrial complexes I and II, also known as NADH dehydrogenase and succinate dehydrogenase, respectively, and mitochondrial complex III, also known as cytochrome bc1 complex. CoQ10 is also reduced to ubiquinol by ferroptosis suppressor protein 1 (FSP1) with NADPH as a cofactor, and ubiquinol traps lipid peroxyl radicals and inhibits lipid peroxidation helping to prevent ferroptosis. Mutations in genes encoding enzymes involved in CoQ10 biosynthesis lead to primary CoQ10 deficiency, which is characterized by encephalopathy, cerebellar ataxia, infantile multisystemic form, nephropathy, and isolated myopathy. Secondary CoQ10 deficiency, induced by non-genetic impaired CoQ10 biosynthesis, insufficient CoQ10 intake, or excessive CoQ10 utilization, has been observed in a variety of conditions, including ataxia-oculomotor-apraxia 1 (AOA1), mitochondrial diseases, and hypercholesteremia with statin therapy. Formulations containing CoQ10 have been used in the treatment of CoQ10 deficiency. | | 化学的特性 | Yellow-Orange Crystalline Powder | | Originator | Neuquinon,Eisai,Japan,1974 | | 天然物の起源 | Coenzyme Q10 is found in dietary sources. Coenzyme Qmolecules have side chains of differentlengths in different types of organismsbut function in similar ways. | | 使用 | Coenzyme Q10 (CoQ10) is a naturally occurring quinone found throughout the body in cell membranes, primarily in mitochondrial membranes, with highest concentrations in the heart, lungs, liver, kidneys, spleen, pancreas, and adrenal glands. It is a component of the electron transport chain and participates in aerobic cellular respiration, generating energy in the form of ATP. In its reduced form, CoQ10 acts as an antioxidant, preventing the formation of reactive oxygen species. CoQ10 deficiencies have been associated with heart failure, hypertension, parkinsonism, mitochondrial encephalomyopathies, and other chronic diseases.[Cayman Chemical] | | 使用 | Ubidecarenone is also known as coenzyme Q10 it is a powerful antioxidant that is naturally found in the cells. It acts as a free radical neutraliser. | | 定義 | ChEBI: Coenzyme Q10 is a ubiquinone having a side chain of 10 isoprenoid units. In the naturally occurring isomer, all isoprenyl double bonds are in the E- configuration. It has a role as a human metabolite, a ferroptosis inhibitor and an antioxidant. It is found in animal organs and yeast with active in the citric acid cycle in carbohydrate metabolism. | | Manufacturing Process | A small fermentation tank (5,000 parts by volume capacity) was charged with
3,000 parts by volume of a culture medium (pH 6.0) comprising 3% glucose,
1% polypepton, 0.5% yeast extract and 0.5% malt extract. The medium was
sterilized by heating in a conventional manner and cooled. This medium was
inoculated with 150 parts by volume of a pre-culture of Sporidiobolus ruinenii
CBS-5001, which had been prepared by growing the same strain on a medium
of the same composition as above at 28°C for one day. The inoculated
medium was incubated at 28°C and under agitation at 800 rpm with sparging
at a rate of 3,000 parts by volume per minute for 24 hours. During this
fermentation period, the medium was maintained at pH 6.0 with ammonia and
sulfuric acid.
The resultant fermentation broth was centrifuged to harvest the microbial
cells, and they were washed with water and centrifuged a second time,
whereupon a living cell paste was obtained. (There was obtained an amount
of cells equivalent to 54 parts on a dry basis, which contained 920 μg of
ubiquinone-10 per gram of dry cells.)
The moist cells were suspended in 750 parts of volume of ethanol and
extracted by warming at 60°C for 1 hour. A total of 3 extractions were carried
out in a similar manner and the extracts were pooled, diluted with water and
further extracted three times with 1,000 parts of volume portions of n_x0002_hexane. The n-hexane layer was concentrated to dryness under reduced
pressure to recover 4.12 parts of a yellow oil. This oily residue was dissolved
in 6 parts by volume of benzene and passed through a column (500 parts by
volume capacity) packed with Floridil (100 to 200 meshes). Elution was
carried out using benzene and the eluate was collected in 10 parts by volume
fractions. Each fraction was analyzed by thin-layer chromatography and color
reaction and the fractions rich in ubiquinone-10 were pooled and concentrated
under reduced pressure. By this procedure was obtained 0.562 part of a
yellow oil. This product was dissolved in 5 parts by volume of chloroform,
coated onto a thin layer plate of silica gel GF254 (silica gel with calcium
sulfate) and developed with benzene. The fractions corresponding to
ubiquinone-10 were extracted, whereby 0.054 part of a yellow oil was
obtained. This oil was dissolved in 10 parts by volume of ethanol and allowed
to cool, whereupon 0.029 part of yellow crystals of ubiquinone-10 were
obtained, its melting point 48° to 50°C.
There are also synthetic routes to the ubiquinones as described in US Patents
3,068,295; 3,896,153 and 4,062,879. | | Therapeutic Function | UBIDECARENONE | | 一般的な説明 | Ubidecarenone, referred to as coenzyme Q, is abundantly available in human cells, with major proportions being present in vital body organs such as heart and kidneys. It plays an important role in cell metabolism owing to its significant contribution to the electron transport pathway. It is a strong antioxidant and acts as an essential enzyme catalyst in mitochondrial oxidative phosphorylation. | | 生物活性 | Component of the mitochondrial transporter chain that behaves as a powerful antioxidant. Displays neuroprotective activity. | | Biochem/physiol Actions | Coenzyme Q10 is an endogenous cellular antioxidant and an essential component of the electron transfer chain. CoQ10 takes part in aerobic cellular respiration and generation of energy in the form of ATP. | | 副作用 | Mild side effects might include digestive problems such as:
Upper abdominal pain; Loss of appetite; Nausea and vomiting; Diarrhea.
Other possible side effects may include:
Headaches and dizziness; Insomnia; Fatigue; Skin itching or rashes; Irritability or agitation. | | 薬物相互作用 | Coenzyme Q10 supplements may interact with some antihypertensive (those that lower blood pressure) and chemotherapy drugs. Coenzyme Q10 may increase the risk of blood clots in people who take the anticoagulant warfarin by decreasing warfarin's effectiveness. | | 純化方法 | Purify it by recrystallisation from EtOH, EtOH/Me2CO or Et2O/EtOH and by chromatography on silica gel using isoPrOH/Et2O (3:1) to give orange crystals. It has max 270nm (� 15,170) in pet ether. [Terao et al. J Org Chem 44 868 1979, NMR and MS: Naruta et al. J Org Chem 45 4097 1980, IR: Lester et al. Biochem Biophys Acta 42 1278 1959, NMR: Planta et al. Helv Chim Acta 42 1278 1959; Morton Biochemical Spectroscopy (Adam Hilger, London, 1975) p 491]. |
|