|
ChemicalBook Optimization Suppliers |
|
| 融点 | 1840 °C | | 沸点 | 2900 °C | | 比重(密度) | 4.26 g/mL at 25 °C(lit.) | | 屈折率 | 2.61 | | 闪点 | 2500-3000°C | | 貯蔵温度 | Store at +5°C to +30°C. | | 溶解性 | Practically insoluble in water. It does not dissolve in dilute mineral acids but dissolves slowly in hot concentrated sulfuric acid. | | 外見 | powder | | 色 | White to slightly yellow | | 比重 | 4.26 | | PH | 7-8 (100g/l, H2O, 20℃)(slurry) | | 臭い (Odor) | at 100.00?%. odorless | | 水溶解度 | insoluble | | Crystal Structure | Orthorhombic, Pcab | | Merck | 14,9472 | | 暴露限界値 | ACGIH: TWA 10 mg/m3
OSHA: TWA 15 mg/m3
NIOSH: IDLH 5000 mg/m3; TWA 2.4 mg/m3; TWA 0.3 mg/m3 | | Dielectric constant | 2.9(20℃) | | CAS データベース | 13463-67-7(CAS DataBase Reference) | | IARC | 2B (Vol. 47, 93) 2010 | | NISTの化学物質情報 | Titanium dioxide(13463-67-7) | | EPAの化学物質情報 | Titanium dioxide (13463-67-7) |
| | 二酸化チタン Usage And Synthesis |
| 種類 | チタニアは結晶構造の違いから、アナターゼ型、ルチル型、ブルッカイト型の3種類が存在します。アナターゼ型とルチル型は正方晶で、ブルッカイト型は斜方晶です。
アナターゼ型を900℃以上に、ブルッカイト型を650℃以上に熱すると、ルチル型に転移します。最安定構造はルチル型です。そのため、ルチル型に一度転移すると、低温に戻しても構造を維持します。工業用に用いられている結晶構造は、ルチル型とアナターゼ型です。
触媒としての活性が低く、熱安定性にも優れる構造であるルチル型は顔料として使用されることが多いです。また、アナターゼ型の方がバンドギャップが大きいため、一般的に光触媒としての活性が高く、光触媒としてはアナターゼ型が使用されることが多いです。
| | 性質 | チタニアは、フッ化水素酸、熱濃硫酸および溶融アルカリ塩に溶解します。一方、その他の酸、アルカリ、水および有機溶剤には溶解しません。
またアナターゼ型とルチル型で密度が異なり、アナターゼ型が3.78g/cm3、ルチル型が4.23g/cm3とルチル型がかなり重くなっています。
| | 性質 | 酸化チタンは、熱濃硫酸、フッ化水素酸、溶融アルカリ塩などに溶解しますが、などの酸には不溶です。アルカリ、水、有機溶剤にも溶けません。
酸化チタンの屈折率は、ダイヤモンドよりも高いです。光触媒作用を有しており、光を受けると表面で強力な酸化力が生じます。
| | 定義 | 本品は、チタンの酸化物であり、次の化学式で表される。 | | 解説 | TiO2(79.87).3種類の結晶形が存在し,天然にも3種類の変態,ルチル(rutil),鋭すい石(anatase),板チタン石(brookite)として産出するが,ルチルがもっとも普通である.いずれも融点1640 ℃.3000 ℃ 以上で分解する.人工的には,四価のチタン塩水溶液の加水分解により得られた含水酸化物を強熱するか,四塩化チタンを高温気体状態で酸素と反応させると得られる.含水酸化物を焼くことにより得られるものは,通常,鋭すい石型であるが,高温で焼くとルチル型となる.四塩化チタンと酸素との反応で得られるものはルチル型である.密度4.26 g cm-3.二酸化チタンはアルカリ,硫酸に可溶,冷水,熱水,その他の酸に不溶.微粉末状の二酸化チタンはチタン白の名の顔料として,塗料,ゴム,繊維,樹脂,磁器,研磨剤,金属チタンの製造原料,化粧品,紫外線防止剤,光触媒,光電極などに広く利用されており,天然産,あるいは人工的に粉末状の二酸化チタンを溶融してつくった塊状の二酸化チタンは,チタニアという名で宝石として使用されている.[CAS 13463-67-7:TiO2(ルチル)][CAS 1317-70-0:TiO2(アナターゼ)]
森北出版「化学辞典(第2版) | | 解説 | 二酸化チタンは,チタンの酸化物の一、酸化チタン(Ⅳ)。チタンの酸化物の中で最も安定で、天然には金紅石きんこうせき(正方晶系)、鋭錐石えいすいせき(正方晶系)、板チタン石(斜方晶系)などの鉱物として産する。酸化チタン。チタニア。チタンと酸素の化合物。酸化チタン(Ⅳ)とも、チタニアtitaniaともよばれる。チタン(Ⅳ)塩水溶液の加水分解で沈殿した水酸化チタン(Ⅳ)を強熱すると得られる。天然にはルチル(金紅石、正方晶系)、鋭錐石(えいすいせき)、板チタン石(斜方晶系)のそれぞれ結晶構造の異なる鉱物として産出する。アルカリと硫酸には溶けるが、それ以外の酸と水には溶けない。藤嶋昭(1942― )と本多健一(1925―2011)による二酸化チタン電極光触媒効果(本多・藤嶋効果)の発見(1968)から、光触媒としての開発研究が活発に続けられている。[岩本振武] | | 用途 | 塗料?印刷インキ?インクジェットインキ?プラスチックの着色顔料,化粧品?シリコーンゴム?プラスチック繊維?磁気テープ?トナー?セラミックスなどの配合原料 (NITE CHRIP) | | 構造 | 酸化チタン (IV) には、アナターゼ型、ルチル型、ブルッカイト型の結晶構造が存在します。アナターゼ型とルチル型は正方晶で、ブルッカイト型は斜方晶です。
アナターゼ型を900℃以上に、ブルッカイト型を650℃以上に熱すると、ルチル型に転移します。最安定構造はルチル型です。そのため、ルチル型に一度転移すると、低温に戻しても構造を維持します。
工業用に用いられている結晶構造は、ルチル型とアナターゼ型です。屈折率などの性質や用途が異なります。天然で酸化チタン (IV) は、金紅石、鋭錐石、板チタン石の主成分として産出します。金紅石と鋭錐石は正方晶系で、板チタン石は斜方晶系の結晶構造です。
| | 製造 | 原料としてルチル鉱石やイルメナイト鉱石 (FeTiO3) が使われます。工業的生産のための主な方法は、塩素法 (英: chlorine method) と硫酸法 (英: sulfuric acid method) です。
塩素法は気相法 (英: gas phase method) とも呼ばれています。まず、ルチル鉱石をコークスやと反応させて、ガス状の四塩化チタンにします。その後冷却して液状にし、高温で酸素と反応させて、塩素ガスを分離することで酸化チタンを生成可能です。
硫酸法は液相法 (英: liquid‐phase method) とも呼ばれます。イルメナイト鉱石を濃硫酸に溶かして、不純物をとして分離し、オキシ硫酸チタンにします。加水分解によってオキシ水酸化チタンが沈殿し、洗浄、乾燥、焼成により酸化チタンを得ることが可能です。 | | 化粧品の成分用途 | 不透明化剤、褪色防止剤、着色剤、紫外線吸収剤.散乱剤 | | 効能 | 皮膚保護薬 | | 主な用途/役割 | ポリウレタン系接着剤に使用される。 | | 説明 | Titanium dioxide, TiO2, is a white powder and has the greatest hiding power of all white pigments. It is noncombustible; however, it is a powder and, when suspended in air, may cause a dust explosion if an ignition source is present. It is not listed in the DOT Hazardous Materials Table, and the DOT does not consider it hazardous in transportation. The primary uses are as a white pigment in paints, paper, rubber, and plastics; in cosmetics; in welding rods; and in radioactive decontamination of the skin. | | 化学的特性 | White, amorphous, odorless, and tasteless nonhygroscopic powder.
Although the average particle size of titanium dioxide powder is less
than 1 mm, commercial titanium dioxide generally occurs as
aggregated particles of approximately 100 mm diameter.
Titanium dioxide may occur in several different crystalline
forms: rutile; anatase; and brookite. Of these, rutile and anatase are
the only forms of commercial importance. Rutile is the more
thermodynamically stable crystalline form, but anatase is the form
most commonly used in pharmaceutical applications. | | 化学的特性 | Ttitanium dioxide is an odorless white powder. | | 化学的特性 | The naturally occurring dioxide exists in three crystal forms: anatase, rutile and brookite. While rutile, the most common form, has an octahedral structure. Anatase and brookite have very distorted octahedra of oxygen atoms surrounding each titanium atom. In such distorted octahedral structures, two oxygen atoms are relatively closer to titanium than the other four oxygen atoms. Anatase is more stable than the rutile form by about 8 to 12 kJ/mol (Cotton, F.A., Wilkinson, G., Murillo, C.A and M Bochmann. 1999. Advanced Inorganic Chemistry, 6th ed, p. 697, New York: John Wiley & Sons) Other physical properties are: density 4.23g/cm3; Mohs hardness 5.8 g/cm3 ( anatase and brookite) and 6.2 g/cm3 ( rutile); index of refraction 2.488 (anatase), 2.583 (brookite) and 2.609 (rutile); melts at 1,843°C; insoluble in water and dilute acids; soluble in concentrated acids. | | 物理的性質 | Metastable over long periods of
time despite being less
thermodynamically stable than
rutile. However, above 700°C,
the irreversible and rapid
monotropic conversion of
anatase to rutile occurs. It
exhibits a greater transparency
in the near-UV than rutile. With
an absorption edge at 385 nm,
anatase absorbs less light at the
blue end of the visible spectrum
and has a blue tone. | | 物理的性質 | The naturally occurring dioxide exists in three crystal forms: anatase, rutile and brookite. While rutile, the most common form, has an octahedral structure. Anatase and brookite have very distorted octahedra of oxygen atoms surrounding each titanium atom. In such distorted octahedral structures, two oxygen atoms are relatively closer to titanium than the other four oxygen atoms. Anatase is more stable than the rutile form by about 8 to 12 kJ/mol (Cotton, F.A., Wilkinson, G., Murillo, C.A and M Bochmann. 1999. Advanced Inorganic Chemistry, 6th ed, p. 697, New York: John Wiley & Sons) Other physical properties are: density 4.23g/cm3; Mohs hardness 5.8 g/cm3 ( anatase and brookite) and 6.2 g/cm3 ( rutile); index of refraction 2.488 (anatase), 2.583 (brookite) and 2.609 (rutile); melts at 1,843°C; insoluble in water and dilute acids; soluble in concentrated acids. | | 天然物の起源 | Titanium dioxide occurs in nature in the crystalline forms rutile, anatase,
and brookite. Rutile and anatase are manufactured in large quantities, which are
primarily used as pigments, but also as catalysts and in ceramics. | | 特性 |
Titanium dioxide (C.I. Pigment White 6) is of outstanding importance as a white pigment because of its scattering properties, its chemical stability, its biological inertness, and its lack of toxicity. The pigment is frequently coated with colorless organic or inorganic compounds of low solubility to improve its weather resistance, lightfastness, and dispersibility.
| | 使用 | Titanium dioxide is an extreme white and bright compound with high index of refraction. In paints it is a white pigment and an opacifying agent.It is in house paints, water paints, lacquers, enamels, paper filling and coating, rubber, plastics, printing ink, synthetic fabrics, floor coverings, and shoe whiteners. Also, it is used in colorants for ceramics and coatings for welding rods. A rutile form of the dioxide is used in synthetic gem stones. | | 使用 | Airfloated ilmenite is used for titanium pigment manufacture. Rutile sand is suitable for welding-rod-coating materials, as ceramic colorant, as source of titanium metal. As color in the food industry. Anatase titanium dioxide is used for welding-rod-coatings, acid resistant vitreous enamels, in specification paints, exterior white house paints, acetate rayon, white interior air-dry and baked enamels and lacquers, inks and plastics, for paper filling and coating, in water paints, tanners' leather finishes, shoe whiteners, and ceramics. High opacity and tinting values are claimed for rutile-like pigments. | | 使用 | Titanium Dioxide is a white pigment that disperses in liquids and
possesses great opacifying power. the crystalline modifications of
titanium dioxide are rutile and anatase, of which only anatase finds
use as a color additive. | | 使用 | titanium dioxide (TiO2) is one of the 21 FDA-approved sunscreen chemicals with an approved usage level of 2 to 25 percent. When applied, titanium dioxide remains on the skin’s surface, scattering uV light. It is often used in conjunction with other sunscreen chemicals to boost the product’s SPF value, thus reducing the risk of irritation or allergies attributed to excessive usage of chemical sunscreens. Its incorporation into sunscreen formulations, makeup bases, and daytime moisturizers depends on the particular size of titanium dioxide employed. The smaller the particle size, the more unobtrusive Tio2’s application. Large particles, on the other hand, leave a whitish wash or look on the skin. Some companies list “micro” or “ultra” when referring to the size of the titanium dioxide particle. According to some sources, titanium dioxide could be the ideal uVA/uVB protection component given its chemical, cosmetic, and physical characteristics. Titanium dioxide is also used to provide a white color to cosmetic preparations. | | 調製方法 | There are two major processes for the manufacture of titanium dioxide
pigments, namely sulfate route and chloride route. In the sulfate
process, the ore limonite, FeOTiO2, is dissolved in sulfuric acid and
the resultant solution is hydrolyzed by boiling to produce a hydrated
oxide, while the iron remains in solution. The precipitated titanium
hydrate is washed and leached free of soluble impurities. Controlled calcinations
at about 1000°C produce pigmentary titanium dioxide of the
correct crystal size distribution; this material is then subjected to a finishing
coating treatment and milling.
The chloride process uses gaseous chlorination of mineral rutile, followed
by distillation and finally a vapor phase oxidation of the titanium
tetrachloride. | | 調製方法 | Titanium dioxide occurs naturally as the minerals rutile (tetragonal
structure), anatase (tetragonal structure), and brookite (orthorhombic
structure).
Titanium dioxide may be prepared commercially by either the
sulfate or chloride process. In the sulfate process a titanium
containing ore, such as ilemenite, is digested in sulfuric acid. This
step is followed by dissolving the sulfates in water, then precipitating
the hydrous titanium dioxide using hydrolysis. Finally, the
product is calcinated at high temperature. In the chloride process,
the dry ore is chlorinated at high temperature to form titanium
tetrachloride, which is subsequently oxidized to form titanium
dioxide. | | 定義 | ChEBI: Titanium dioxide is a titanium oxide with the formula TiO2. A naturally occurring oxide sourced from ilmenite, rutile and anatase, it has a wide range of applications. It has a role as a food colouring. | | 主な応用 |
Industry
Application
Role/benefit
Pigment
Optical coating for dielectric mirrors and gemstones
Brightness and very high refractive index
Paper coating
Helps to make paper whiter, brighter and more opaque
Plastics, adhesives and rubber
Helps minimize the brittleness, fading and cracking that can occur as a result of light exposure
Food Contact materials and ingredients
Prevents premature degradation and enhance the longevity of the product
Paints
Gives paint its high gloss and rich depth of color
Ceramic glazes
Acts as an opacifier and seeds crystal formation
Cosmetic
Sunscreens
Active ingredients/high refractive index and strong UV light absorbing capabilities
Daily cosmetics or make-up materials
Additive/aids in hiding blemishes and brightening the skin
Toothpastes
Additive/helps to whiten tooth
Catalyst
Dye-sensitized solar cell
Can produce electricity in nanoparticle form
Hydrolysis reaction
Catalyzes the photo decomposition of water into hydrogen and oxygen
Automotive, power stations, etc.
Helps to removes harmful exhaust gas emissions, such as nitrous oxides, volatile organic compounds, etc.
Detoxification or remediation of wastewater
Photocatalytically mineralizes pollutants (to convert into CO2 and H2O) in waste water
Photocatalytic antimicrobial coating
Photocatalytic destruction of organic matter
Others
Oxygen sensor
The electrical resistivity of TiO2 can be correlated to the oxygen content of the atmosphere
Anti-fogging coatings and self-cleaning windows
Under exposure to UV light, TiO2 becomes increasingly hydrophilic
Coated ceramic tile
Disinfectant and self-cleaning qualities
Treatment of the air in fruit, vegetable and cut flower storage areas
Removes ethylene gas to prevent spoilage and prevents internal combustion
Memristor
Can be employed for solar energy conversion
Mixed conductor
Significant ionic and electronic conduction
| | 製造方法 | Titanium dioxide is mined from natural deposits. It also is produced from other titanium minerals or prepared in the laboratory. Pigment-grade dioxide is produced from the minerals, rutile and ilmenite. Rutile is converted to pigment grade rutile by chlorination to give titanium tetrachloride, TiCl4. Anhydrous tetrachloride is converted back to purified rutile form by vapor phase oxidation.
Anatase form is obtained by hydrolytic precipitation of titanium(IV) sulfate on heating. The mineral ilmenite is treated with concentrated sulfuric acid. Heating the sulfate solution precipitates hydrous titanium oxide. The precipitate is calcined to expel all water.
Titanium dioxide also can be prepared by heating Ti metal in air or oxygen at elevated temperatures. | | Composition | This material is visually a brilliant white pigment which also has anti-inflammatory properties. Two crystal types of TiO2 occur: anatase and rutile. In order to produce these crystals, there are two manu�facturing processes that are employed: (1) The sulfate manufacturing process has the ability to produce either type of crystal, while (2) the chloride manufacturing process produces only rutile crystals. | | 一般的な説明 | Two main physico-chemically distinct polymorphs of TiO2 are anatase and rutile. Anatase has a higher photocatalytic activity than rutile but is thermodynamically less stable. | | 危険性 | Lower respiratory tract irritant. Possible
carcinogen. | | 健康ハザード | Titanium dioxide is a mild pulmonary
irritant and is generally regarded as a
nuisance dust. | | 燃焼性と爆発性 | Not classified | | 使用用途 | 酸化チタンは、優れた白色度や隠蔽力、着色力、化学的に極めて高い安定性などの特色を活かし、白色顔料として、塗料や絵具、釉薬、印刷インキ、化合繊等の用途で幅広く使用されています。その他、酸化チタンの光触媒作用を利用して、工業的に難分解性の物質を分解しています。
酸化チタンは、安全な着色料としての用途や、紫外線防御作用の目的から、日焼け止め製品や化粧品、洗顔料・洗顔石鹸、ネイル製品等にも使用されています。
| | 使用用途 | チタニアの主な使用用途は、顔料および光触媒です。チタニアは国内で約12万トンが毎年生産されています。
顔料や白色の着色剤の原料として使用されます。チタン白、チタンホワイト、チタニウムホワイトと呼ばれ、高い隠蔽力をもちます。顔料としての分類名 (カラーインデックス名) は、C.I.Pigment White 6です。チタニアは、優れた白色度や隠蔽力、着色力、化学的に極めて高い安定性などの特色を活かし、白色顔料として、塗料や絵具、インクジェットインキ、プラスチックの着色顔料、釉薬、印刷インキ、化繊等の用途で幅広く使用されています。
また、チタニアは紫外線相当の短波長の光を受けると、水と反応して活性酸素種を生成します。このような光により、表面で強力な触媒能力を発現する物質を光触媒と呼びます。活性酸素種は、強い酸化力をもつため、化学薬品や細菌などに対して分解作用を示します。その他、チタニアの光触媒作用を利用して、工業的に難分解性の物質を分解しています。
人体への影響が小さいため、食品・医薬品・化粧品の着色料 (食品添加物) としても有用です。チタニアの人工結晶は、無色透明であり、屈折率がタイヤモンドよりも高いため、人工宝石としての用途もあります。シリコーンゴム・・セラミックスの配合原料、オフセット印刷の感光体、固体触媒の担体、日焼け止め製品、化粧品、洗顔料・洗顔石鹸、ネイル製品等にも使用されています。
| | 化学性质 | 白色固体で,水や希酸に不溶,濃硫酸や硫酸水素カリウムには徐々に溶解する。 | | 還元 | 600℃以上で酸化チタン (IV) は、水素ガスによって部分的に還元されて、青色の (III) が混じった酸化物を生成します。ただし、酸素に触れると、速やかに酸化チタン (IV) に戻ります。
酸化チタン (IV) に担持された貴金属触媒を高温で還元すると、SMSI (英: Strong Metal Support Interaction) が発生しやすいです。SMSIとは、酸化物担体に担持した金属ナノ粒子が反応ガスに触れた際に、触媒の活性が大きく変化する現象のことです。
900℃以上で水素還元した場合には、濃青色で不定比組成のTiOx (x=1.85〜1.94) を生成します。この組成は常温常圧で酸素に触れた際にも安定しています。
参考文献
| | 応用例(製薬) | Titanium dioxide is widely used in confectionery, cosmetics, and
foods, in the plastics industry, and in topical and oral pharmaceutical
formulations as a white pigment.
Owing to its high refractive index, titanium dioxide has lightscattering
properties that may be exploited in its use as a white
pigment and opacifier. The range of light that is scattered can be
altered by varying the particle size of the titanium dioxide powder.
For example, titanium dioxide with an average particle size of
230nm scatters visible light, while titanium dioxide with an average particle size of 60nm scatters ultraviolet light and reflects visible
light.
In pharmaceutical formulations, titanium dioxide is used as a
white pigment in film-coating suspensions, sugar-coated tablets,
and gelatin capsules. Titanium dioxide may also be admixed with
other pigments.
Titanium dioxide is also used in dermatological preparations
and cosmetics, such as sunscreens. | | 安全性プロファイル | A nuisance dust. A
human skin irritant. Questionable
carcinogen with experimental carcinogenic,
neoplastigenic, and tumorigenic data.
Violent or incandescent reaction with metals
at high temperatures (e.g., aluminum,
calcium, magnesium, potassium, sodium,
zinc, lithium). See also TITANIUM
COMPOUNDS. | | 安全性 | Titanium dioxide is widely used in foods and oral and topical
pharmaceutical formulations. It is generally regarded as an
essentially nonirritant and nontoxic excipient. | | 職業ばく露 | Titanium dioxide is a white pigment used as a pigment in paint; in the rubber, plastics, ceramics, paint, and varnish industries, in dermatological preparations; and is used as a starting material for other titanium compounds; as a gem; in curing concrete; and in coatings for welding rods. It is also used in paper and cardboard manufacture. | | 概要 | チタニアとは、化学式TiO2で表されるチタンの酸化物です。
チタニアはの酸化物の中で最も安定な化合物です。別称としては、酸化チタン (Ⅳ) のほか、二酸化チタン、ジオキソチタン (IV) 、チタン (IV) ジオキシドなどがあります
| | 発がん性 | Carcinogenesis. In a 1985 study, rats (CD) were
exposed to graded airborne concentrations (0, 10, 50, and
250mg/m3) of TiO2 6 h/day, 5 days/week, for 2 years. The
majority of the particles were in the respirable range (84%
≤13 mmMMD). All responses were confined to the lungs. At
the lowest dose, the histopathological evaluation of the lungs
revealed dust-laden macrophages in the alveolar ducts and
adjacent alveoli with pneumocyte hyperplasia. At the two
highest concentrations, there were increases in lung weight,
accumulation of dust in the macrophages, foamy macrophage
responses, type II pneumocyte hyperplasia, alveolar proteinosis,
alveolar bronchiolization, cholesterol granulomas, focal
pleurisy, and dust deposition in the tracheobronchiolar lymph
nodes. At the 250mg/m3 exposure concentration, bronchiole
alveolar adenomas (males: control 2/79, 250mg/m3 12/79;
females: control 0/79, 250mg/m3 13/79) increased.
Additionally, 13/79 females at the 250mg/m3 dose showed squamous cell carcinoma, compared with none in 79 controls.
Theauthorsnoted that this responsemight have little biological
relevance to humans because of the overload of respiratory
clearance mechanisms and also pointed out that the type,
location, and development of the tumors were different from
those in human lung tumors. It is not clear that the nasal
cavity epithelium was examined. However, the nasal cavity
load would be expected to be higher in the rats because of
anatomic structure, whereas the lung deposition should be
higher in humans because we are, in part, mouth breathers. | | 製造方法 | 天然には、高温で生成される火成岩、変成岩の副成分鉱物として分布し、金紅石 (ルチル型) 、鋭錐石 (アナターゼ型) 、板チタン石などの鉱物として産出されます。チタニアの工業的生産では原料にイルメナイト鉱石 (FeTiO3) または、ルチル鉱石が用いられます。硫酸法または塩素法の2つの方法で製造されています。
1. 硫酸法 (イルメナイト鉱石から)
硫酸法では、イルメナイト鉱石を濃硫酸に溶解させ、鉄分を硫酸鉄として分離し、オキシ硫酸チタンに変換します。それを加水分解してオキシ水酸化チタン (TiO(OH)2) にし、沈殿させてチタニアを得ます。
2. 塩素法 (ルチル鉱石から)
塩素法では、ルチル鉱石をコークス・塩素と反応させ、四塩化チタンを作ります。その後、高温で酸素と反応させ、塩素ガスを分離回収してチタニアを得ます。 | | 貯蔵 | Titanium dioxide is extremely stable at high temperatures. This is
due to the strong bond between the tetravalent titanium ion and the
bivalent oxygen ions. However, titanium dioxide can lose small,
unweighable amounts of oxygen by interaction with radiant energy.
This oxygen can easily recombine again as a part of a reversible
photochemical reaction, particularly if there is no oxidizable
material available. These small oxygen losses are important because
they can cause significant changes in the optical and electrical
properties of the pigment.
Titanium dioxide should be stored in a well-closed container,
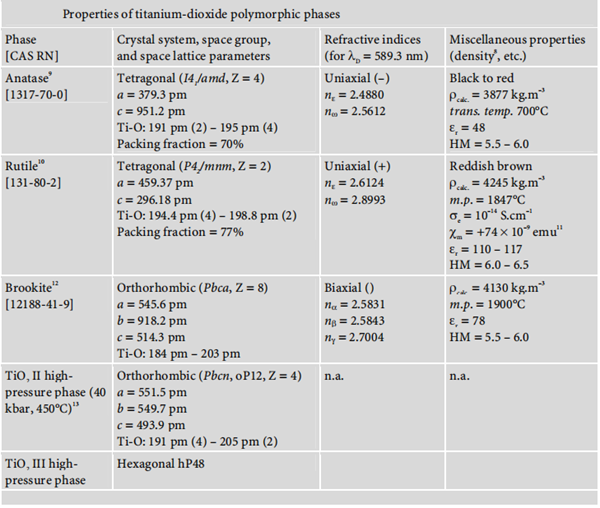
protected from light, in a cool, dry place. | | 合成方法 | 通过钛的氧化物反应制得 | | Structure and conformation | Rutile and anastase crystals are tetragonal. Rutile crystals have greater coverage due to the close packing orientation of the atoms in the crystal. The refractive indices for anatase and rutile crystals are 2.55 and 2.71, respectively. The resultant opacity is due to the light scattering ability of the TiO2. Light, heat, and chemical stability are excellent when employing this material. Additionally, in the United States, TiO2 is regarded as a Category I sunscreen. | | Forms and nomenclature | Titanium dioxide occurs in nature in three polymorphic crystal forms: anatase, rutile, and brookite.
Moreover, under high pressure, the structure of all three polymorphs of titanium dioxide
may be converted into that of α-PbO2. The following diagram summarises the main properties of these three polymorphisms:
 | | 不和合性 | Titanium dioxide is incompatible with strong oxidizers and strong acids. Violent or incandescent reactions may occur with metals (e.g., aluminum, calcium, magnesium, potassium, sodium, zinc, and lithium). | | 不和合性 | Owing to a photocatalytic effect, titanium dioxide may interact
with certain active substances, e.g. famotidine. Studies have shown that titanium dioxide monatonically degrades film mechanical
properties and increases water vapor permeability of polyvinyl
alcohol coatings when used as an inert filler and whitener.
Titanium dioxide has also been shown to induce photooxidation
of unsaturated lipids. | | 廃棄物の処理 | Land fill. | | 規制状況(Regulatory Status) | Accepted as a food additive in Europe. Included in the FDA Inactive
Ingredients Database (dental paste; intrauterine suppositories; ophthalmic preparations; oral capsules, suspensions, tablets; topical
and transdermal preparations). Included in nonparenteral medicines
licensed in the UK. Included in the Canadian List of
Acceptable Non-medicinal Ingredients. |
|