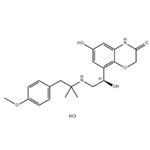
- Olodaterol Hydrochloride
-
- $300.00 / 1kg
-
2024-04-26
- CAS:869477-96-3
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: 200kg per month
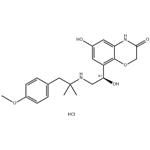
- BI 1744 hydrochloride
-
- $6.00 / 1KG
-
2024-03-29
- CAS:869477-96-3
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
- olodaterol hydrochloride
-

-
2021-07-09
- CAS:869477-96-3
- Min. Order: 1KG
- Purity: 99% +
- Supply Ability: 500 Ton/Tons per Month
|
| | BI 1744 hydrochloride Basic information |
| Product Name: | BI 1744 hydrochloride | | Synonyms: | Olodaterol HCl;BI 1744 hydrochloride;Olodaterol hydrochloride;Olodaterol(BI-1744) HCl;Olodaterol(BI-1744) hydrochloride;BI-1744;BI 1744;BI1744;2H-1,4-Benzoxazin-3(4H)-one, 6-hydroxy-8-[(1R)-1-hydroxy-2-[[2-(4-methoxyphenyl)-1,1-dimethylethyl]amino]ethyl]-, hydrochloride (1:1);BI-1744 HCl | | CAS: | 869477-96-3 | | MF: | C21H27ClN2O5 | | MW: | 422.90248 | | EINECS: | 000-000-0 | | Product Categories: | | | Mol File: | 869477-96-3.mol | 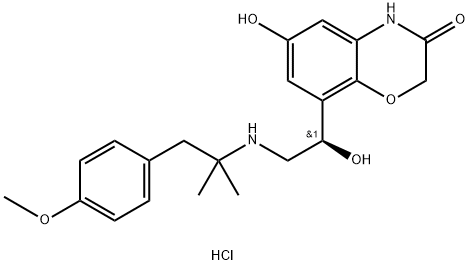 |
| | BI 1744 hydrochloride Chemical Properties |
| Melting point | 153-155°C | | storage temp. | Hygroscopic, -20°C Freezer, Under inert atmosphere | | solubility | DMSO (Slightly), Methanol (Slightly) | | form | Solid | | color | Off-White to Pale Beige | | Water Solubility | Water : 250 mg/mL (591.16 mM; Need ultrasonic) | | Stability: | Hygroscopic |
| | BI 1744 hydrochloride Usage And Synthesis |
| Description | Olodaterol hydrochloride was approved for long-term, oncedaily
maintenance treatment of chronic obstructive pulmonary
disease (COPD) in 2013 in the following countries: Canada,
Russia, United Kingdom, Denmark, and Iceland. The drug
has been recommended by a federal advisory panel for approval
by the FDA. Developed and marketed by Boehringer
Ingelheim, olodaterol is a long-acting b2-adrenergic receptor agonist
with high selectivity over the b1- and b3-receptors (219- and
1622-fold, respectively). Upon binding to and activating the
b2-adrenergic receptor in the airway, olodaterol stimulates adenyl
cyclase to synthesize cAMP, leading to the relaxation of smooth
muscle cells in the airway. Administered by inhalation using the
Respimat® Soft Mist inhaler, it delivers significant bronchodilator
effects within five minutes of the first dose and provides sustained
improvement in forced expiratory volume (FEV1) for over 24 h. | | Uses | Olodaterol is a long acting β-adrenoceptor agonist used as an inhalation for treating patients with chronic obstructive pulmonary disease (COPD). | | Definition | ChEBI: A hydrochloride obtained by combining olodaterol with one equivalent of hydrochloric acid. Used for long-term treatment of airflow obstruction in patients with chronic obstructive pulmonary disease including chronic bronchitis and/or emphysema. | | Synthesis | Commercial 20,50-dihydroxyacetophenone (122) was treated
with one equivalent of benzyl bromide and potassium carbonate
in methylisobutylketone (MIBK) to give the 50-monobenzylated
product in 76% yield. Subsequent nitration occurred at the 40-position
to provide nitrophenol 123 in 87% yield. Reduction of the nitro
group followed by subjection to chloroacetyl chloride resulted in
the construction of benzoxazine 124 in 82% yield. Next, monobromination
through the use of tetrabutylammonium tribromide
occurred at the acetophenone carbon to provide bromoketone
125, and this was followed by asymmetric reduction of the ketone
employing (�)-DIP chloride to afford an intermediate bromohydrin,
which underwent conversion to the corresponding
epoxide 126 in situ upon treatment with aqueous NaOH. This
epoxide was efficiently formed in 85% yield and 98.3% enantiomeric
excess. Epoxide 126 underwent ring-opening upon subjection
to amine 127 to provide amino-alcohol 128 in in 84¨C90%
yield and 89.5¨C99.5% enantiomeric purity following salt formation
with HCl. Tertiary amine 127 was itself prepared in three steps by
reaction of ketone 129 with methylmagnesium chloride, Ritter
reaction of the tertiary alcohol with acetonitrile, and hydrolysis
of the resultant acetamide with ethanolic potassium hydroxide.
Hydrogenative removal of the benzyl ether within 128 followed
by recrystallization with methanolic isopropanol furnished olodaterol
hydrochloride (XVI) in 63¨C70% yield. Overall, the synthesis
of olodaterol hydrochloride required 10 total steps (7 linear) from
commercially available acetophenone 122. 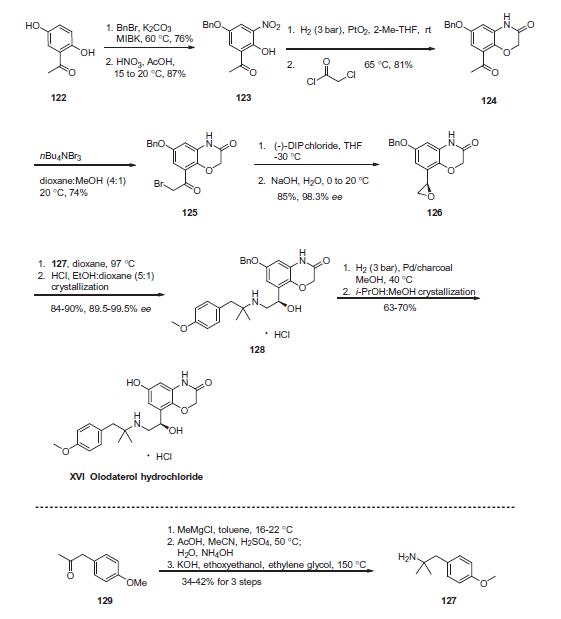
|
| | BI 1744 hydrochloride Preparation Products And Raw materials |
|