- fluspirilene
-
- $6000.00 / 1kg
-
2024-01-18
- CAS:1841-19-6
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 7000
- FLUSPIRILENE USP/EP/BP
-
- $1.10 / 1g
-
2021-07-24
- CAS:1841-19-6
- Min. Order: 1g
- Purity: 99.9%
- Supply Ability: 100 Tons min
|
| | FLUSPIRILENE Basic information |
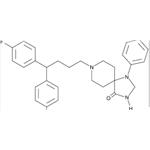
| Product Name: | FLUSPIRILENE | | Synonyms: | R 6218;1-phenyl-4-oxo-8-(4,4-bis(4-fluorophenyl)butyl)-1,3,8-triazaspiro(4,5)decane;8-(4,4-bis(p-fluorophenyl)butyl)-1-phenyl-1,3,8-triazaspiro(4,5)decan-4-one;8-triazaspiro(4.5)decan-4-one,8-(4,4-bis(p-fluorophenyl)butyl)-1-phenyl-3;imap;8-(4,4-BIS[P-FLUOROPHENYL]BUTYL)-1-PHENYL-1,3,8-TRIAZINO(4,5)DECAN-4-ONE;mcn-jr-6218;8-(4,4-bis(4-fluorophenyl)butyl)-1-phenyl-1,3,8-triazaspiro(4.5)decan-4-one | | CAS: | 1841-19-6 | | MF: | C29H31F2N3O | | MW: | 475.57 | | EINECS: | 217-418-6 | | Product Categories: | | | Mol File: | 1841-19-6.mol | 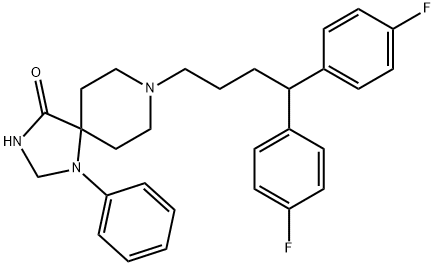 |
| | FLUSPIRILENE Chemical Properties |
| Melting point | 187.5-190° | | Boiling point | 668.9±55.0 °C(Predicted) | | density | 1.1634 (estimate) | | storage temp. | room temp | | solubility | DMSO: soluble | | form | amorphous solid | | pka | 15.05±0.20(Predicted) | | color | white to yellow | | CAS DataBase Reference | 1841-19-6 |
| RIDADR | 3249 | | WGK Germany | 3 | | RTECS | XX8750000 | | HazardClass | 6.1(b) | | PackingGroup | III | | Toxicity | LD50 i.m. in rats: 146 ±14 mg/kg (Janssen) |
| | FLUSPIRILENE Usage And Synthesis |
| Description | Fluspirilene is a potent, non-competitive antagonist of agonist-activated L-type calcium channels (IC50 = 0.03 μM). In addition to its use in research as a calcium channel blocker, fluspirilene has potential application as an antipsychotic in schizophrenia. | | Chemical Properties | White or almost white powder. | | Originator | Imap, Janssen W. ,Germany ,1972 | | Uses | This drug is primarily used for supportive therapy of patients suffering from chronic men�tal illnesses after treatment in the hospital. It is suitable for use in ambulatory practice
because of the lack of expressed hypno-sedative effects. | | Uses | Antipsychotic. | | Uses | Fluspirilene has been used as a neuroleptic drug to study its effects on human ether-a-go-go related gene (HERG). | | Definition | ChEBI: 8-[4,4-bis(4-fluorophenyl)butyl]-1-phenyl-1,3,8-triazaspiro[4.5]decan-4-one is a diarylmethane. | | Manufacturing Process | To a solution of 130 parts cyclopropyl-di-(4-fluorophenyl)-carbinol in 240 parts benzene are added dropwise 43 parts thionylchloride. The whole is refluxed until no more gas is evolved. The reaction mixture is then evaporated. The residue is distilled in vacuo, yielding 4-chloro-1,1-di-(4-fluorophenyl)-1butene, boiling point 165° to 167°C at 6 mm pressure; nD20= 1.5698; d2020= 1.2151.
A solution of 61 parts 4-chloro-1,1-di-(4-fluorophenyl)-1-butene in 400 parts 2-propanol is hydrogenated at normal pressure and at room temperature in the presence of 5.5 parts palladium-on-charcoal catalyst 10% (exothermic reaction, temperature rises to about 30°C). After the calculated amount of hydrogen is taken up, hydrogenation is stopped. The catalyst is filtered off and the filtrate is evaporated. The oily residue is distilled in vacuo, yielding 1chloro-4,4-di-(4-fluorophenyl)-butane, boiling point 166° to 168°C at 6 mm pressure; nD20= 1.5425; d2020= 1,2039.
A mixture of 7.3 parts 1-chloro-4,4-di-(4-fluorophenyl)-butane, 5.1 parts 1phenyl-4-oxo-1,3,8-triaza-spiro[4,5]decane, 4 parts sodium carbonate, a few crystals of potassium iodide in 200 parts 4-methyl-2-pentanone is stirred and refluxed for 60 hours. After cooling the reaction mixture is treated with water. The organic layer is separated, dried, filtered and evaporated. The solid residue is recrystallized from 80 parts 4-methyl-2-pentanone, yielding 1phenyl-4-oxo-8-[4,4-di-(4-fluorophenyl)]butyl-1,3,8-triaza-spiro[4,5]decane, melting point 187.5° to 190°C. | | Brand name | Imap (OrthoMcNeil). | | Therapeutic Function | Tranquilizer | | Biochem/physiol Actions | Fluspirilene has a potential to inhibit the activity of cyclin-dependent kinase 2 (CDK2). It is an effective anti-cancer agent used for treating human hepatocellular carcinoma. | | Synthesis | Fluspirilene, 8-[4,4-bis(p-fluorophenyl)butyl]-1-phenyl-1,3,8-triazaspiro
[4,5]decan-4-one (6.6.9), is synthesized from 1-benzyl-4-anilino-4-cyanopiperidine
(3.1.64) by the way of its acidic hydrolysis into the amide (6.6.6), and the subsequent het�erocyclization of 4-aminocarbonyl and 4-aniline functional groups into imidazolone cycle,
thus creating the desired spiroheterocyclic system, 8-benzyl-1-phenyl-1,3,8-triaza�spiro[4,5] decan-4-one (6.6.7). Hydrogenation of this product using a palladium on carbon
catalyst removes the N-benzyl protecting group, forming 1-phenyl-1,3,8-triazaspiro
[4,5]decan-4-one (6.6.8). Alkylating this with 1,1-bis-(4-fluorophenyl)butyl bromide
(6.6.3) gives fluspirilene (6.6.9) [64¨C66]. 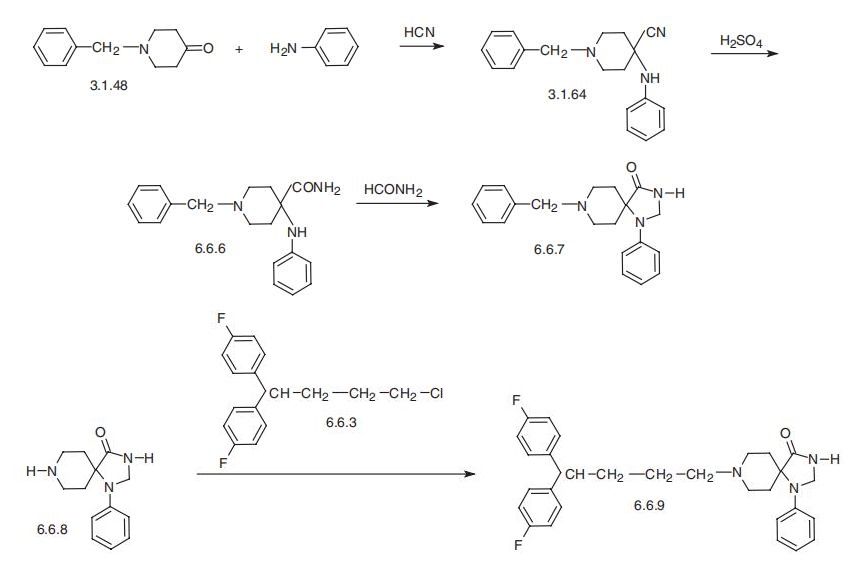
| | in vitro | fluspirilene was found to be weakly active as an antagonist of ca2(+)-induced contractions in k(+)-depolarized taenia. in addition, fluspirilene at 10-1000 nm was a potent non-competitive antagonist of the effects of bay k 8644 on ca2(+)-induced contractions and could selectively antagonise the effects of bay k 8644, without affecting the calcium antagonist effects of nitrendipine [1]. | | in vivo | in a previous animal study, adult male wistar rats were intramuscularly injected with a 8 mg/kg dose of fluspirilene. results showed that the excretion was slow but constant during the first 12 days. the identified metabolites of the urine and faeces showed oxidative n-dealkylation as the major metabolic pathway [2]. | | IC 50 | 0.03 μm | | references | [1] kenny, b. a.,fraser, s.,kilpatrick, a.t., et al. selective antagonism of calcium channel activators by fluspirilene. br. j. pharmacol. 100(2), 211-216 (1990).
[2] heykants jp. the excretion and metabolism of the long-acting neuroleptic drug fluspirilene in the rat. life sci. 1969 oct 1;8(19):1029-39.
[3] chouinard g, annable l, steinberg s. a controlled |
| | FLUSPIRILENE Preparation Products And Raw materials |
|