Alfaxalone manufacturers
- Alfaxalone
-

- $0.00 / 1kg
-
2024-04-02
- CAS:23930-19-0
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20tons
- ALFAXALONE USP/EP/BP
-
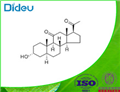
- $1.10 / 1g
-
2021-07-09
- CAS:23930-19-0
- Min. Order: 1g
- Purity: 99.9%
- Supply Ability: 100 Tons Min
|
| | Alfaxalone Basic information |
| Product Name: | Alfaxalone | | Synonyms: | 3ALPHA-HYDROXY-5ALPHA-PREGNANE-11,20-DIONE;3-HYDROXY-5ALPHA-PREGNANE-11,20-DIONE;5ALPHA-PREGNAN-3ALPHA-OL-11,20-DIONE;ALFAXALONE;ALPHAXALONE;(3-alpha,5-alpha)-3-hydroxypregnane-11,20-dione;20-dione,3-alpha-hydroxy-5-alpha-pregnane-1;20-dione,3-hydroxy-,(3-alpha,5-alpha)-pregnane-1 | | CAS: | 23930-19-0 | | MF: | C21H32O3 | | MW: | 332.48 | | EINECS: | | | Product Categories: | | | Mol File: | 23930-19-0.mol | 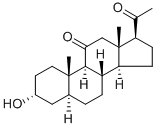 |
| | Alfaxalone Chemical Properties |
| Melting point | 172-174° | | alpha | D26 +113.4° (c = 1.2 in chloroform). | | Boiling point | 409.59°C (rough estimate) | | density | 1.0834 (rough estimate) | | refractive index | 1.5192 (estimate) | | storage temp. | Store at RT | | solubility | chloroform: ≥5 mg/mL, clear, colorless | | form | powder | | pka | 15.05±0.70(Predicted) | | color | white | | InChI | InChI=1/C21H32O3/c1-12(22)16-6-7-17-15-5-4-13-10-14(23)8-9-20(13,2)19(15)18(24)11-21(16,17)3/h13-17,19,23H,4-11H2,1-3H3/t13-,14+,15-,16+,17-,19+,20-,21+/s3 | | InChIKey | DUHUCHOQIDJXAT-YYJIQYHHNA-N | | SMILES | [C@]12([H])C(=O)C[C@]3(C)[C@H](CC[C@@]3([H])[C@]1([H])CC[C@@]1([H])C[C@H](O)CC[C@]21C)C(=O)C |&1:0,5,7,10,12,16,19,23,r| |
| Hazard Codes | Xn | | Risk Statements | 22 | | Safety Statements | 22-36 | | RIDADR | UN 2811 6.1/PG 3 | | WGK Germany | 3 | | RTECS | TU4157800 | | Toxicity | LD50 i.v. in mice: 43 mg/kg (Al-Khawashki) |
| | Alfaxalone Usage And Synthesis |
| Description | Alfaxalone (Alfaxan, Vetoquinol) is a neurologically active steroid compound that induces general anaesthesia. Alfaxalone exerts its action by binding to GABA receptors on the neuronal cell surface, affecting cell membrane chloride ion transport. It has negligible analgesic properties at therapeutic doses. Alfaxalone may be administered either intravenously or intramuscularly, and due to the possibility of apnoea after intravenous induction this route should be reserved for cases where intubation can be achieved[2]. | | Originator | Althesin ,Glaxo ,UK ,1972 | | Uses | Anesthetic;nociception blocker | | Definition | ChEBI: Alphaxolone is a corticosteroid hormone. | | Manufacturing Process | A solution of 3α-hydroxy-5α-pregn-16-ene-11,20-dione (200 mg) in freshly
distilled tetrahydrofuran (8 ml) with 5% palladium on carbon (100 ml) was
hydrogenated until hydrogen uptake ceased. The mixture was filtered through
a pad of kieselguhr and the tetrahydrofuran removed in vacuum to give 196
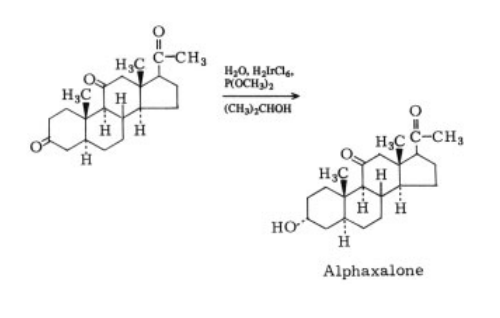
mg, MP 171°C to 172°C. | | Therapeutic Function | Anesthetic component | | Biological Activity | A neurosteroid anesthetic that directly activates and potentiates GABA A receptor-activated membrane current (I GABA ). Efficacy but not potency is determined by the alpha subunit of the receptor (EC 50 values are 1.4, 1.8, 2.1, 2.4 and 2.5 μ M for α 1 β 1 γ 3, α 1 β 1 γ 1, β 1 γ 1, α 2 β 1 γ 2L and α 1 β 1 γ 2L isoforms respectively). | | Mechanism of action | Alfaxalone's mechanism of action is chloride ion modulation associated with interaction with the neuronal membrane GABA A receptor[1]. | | Side effects | Alfaxalone is safe and effective at 12–15 mg/kg IM for routine noninvasive procedures and lasts approximately 40–45 min. The most common side effects are dose-dependent but transient hypotension (15 min), hypoventilation, and apnea. Higher doses of alfaxalone (12 mg/kg) may cause mild hypoxemia, which can be corrected by administration of supplemental oxygen via face mask. Muscle twitching occurs toward the end of immobilization and can be used as a sign of impending emergence[1]. | | Synthesis | It is prepared by
reduction of 3,11,20-trioxo-5-α-pregnane with
trimethyl phosphite in the presence of an iridium
catalyst. Only this reducing agent forms the
axial alcohol (3α) .
 | | References |
[1] Marini, R. and J. Haupt. “Anesthesia and Select Surgical Procedures.” The Common Marmoset in Captivity and Biomedical Research 61 1 (2019).
[2] Molly Varga BVetMed DZooMed MRCVS. “Anaesthesia and Analgesia.” Textbook of Rabbit Medicine (Second Edition) (2014): 178-202.
|
| | Alfaxalone Preparation Products And Raw materials |
|