|
|
| | Boceprevir InterMediates Basic information |
| Product Name: | Boceprevir InterMediates | | Synonyms: | Boceprevir InterMediates;(1S,2S,5R)-Methyl 6,6-diMethyl-3-azabicyclo[3.1.0]hexane-2-carboxylate hydrochloride;3-Azabicyclo[3.1.0]hexane-2-carboxylic acid, 6,6-diMethyl-, Methyl ester, hydrochloride, (1R,2S,5S)-;3-Azabicyclo[3.1.0]hexane-2-carboxylicacid,6,6-diMethyl-,Methylester,hydrochloride(1:1),(1R,2S,5S)-;(1R,2S,5S)-6,6-Dimethyl-3-azabicyclo[3.1.0]hexane-2-carboxylic
acid methyl ester HCl;1.3-Azabicyclo[3.1.0]hexane-2-carboxylic acid, 6,6-dimethyl-, methyl ester, hydrochloride(1R,2S,5S);(1R,2S,5S)-6,6-Dimethyl-3-azabicyclo[3.1.0]hexane-2-carboxylate acid Methyl ester hydrochloridee;(1R,2S,5S)-methyl 6,6-dimethyl-3-aza-bicyclo[3.1.0]hexane-2-... | | CAS: | 565456-77-1 | | MF: | C9H16ClNO2 | | MW: | 205.68 | | EINECS: | 611-400-5 | | Product Categories: | Boceprevir intermediate;Chiral Reagents;Heterocycles;Intermediates & Fine Chemicals;Pharmaceuticals;565456-77-1 | | Mol File: | 565456-77-1.mol | 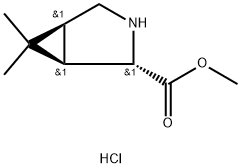 |
| | Boceprevir InterMediates Chemical Properties |
| storage temp. | Inert atmosphere,Room Temperature | | form | solid | | color | Pale yellow | | InChI | InChI=1/C9H15NO2.ClH/c1-9(2)5-4-10-7(6(5)9)8(11)12-3;/h5-7,10H,4H2,1-3H3;1H/t5-,6-,7-;/s3 | | InChIKey | FKVUDBWXNAFSPB-AUBOPKACNA-N | | SMILES | C([C@H]1NC[C@@H]2C(C)(C)[C@H]12)(=O)OC.Cl |&1:1,4,8,r| |
| | Boceprevir InterMediates Usage And Synthesis |
| Uses | (1R, 2S, 5S)-6,6-dimethyl-3-azabicyclo[3.1.0]hexane-2-carboxylate methyl ester hydrochloride is the cost in the side chain synthesis of boceprevir the highest key intermediate. | | Uses | (1S,2S,5R)-Methyl 6,6-diMethyl-3-azabicyclo[3.1.0]hexane-2-carboxylate hydrochloride is an azabicyclohexane derivative used in the preparation of hepatitis C virus (HCV) NS3 serine protease inhibitor. | | General Description | Boceprevir is a peptidomimetic protease inhibitor with four moieties, P1–P3 and a Cap, where P1 is a racemic β-aminoamide, P2 is a chiral dimethylcyclopropylproline analog, P3 is (S)-tert-leucine, and Cap is a tert-butylcarbamoyl group. The 3-azabicyclo[3.1.0]hexane structure of P2 adopts a constrained conformation, so that the gem-dimethyl group has a fixed angle with respect to the bicyclic ring structure. The incorporation of the 3-azabicyclo[3.1.0]hexane moiety results in a 1000-fold increase in NS3 protease binding over proline in a pentapeptide scaffold. When boceprevir binds to the NS3 protease, the P2 moiety interacts with four amino acid residues at the active site. The starting material for the P2 moiety is the HCl salt of the dimethylcyclopropylproline methyl ester 2. For medicinal chemistry studies, it was prepared by cyclopropanation of a Δ3-pyrroline derivative of pyroglutamic acid.
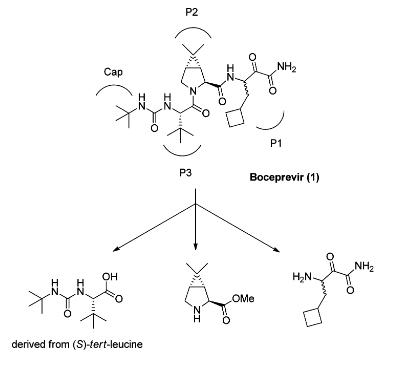 | | Synthesis | 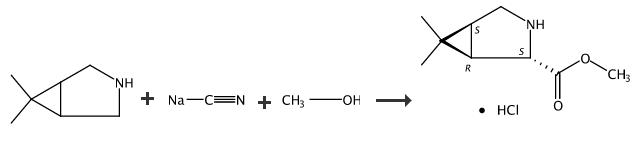
The amine oxidation was carried out in a RC-1 Process Development Workstation (Mettler Toledo) equipped with pH feedback control and a gas induction impeller. A solution of 2.5 g MAON401(Codexis), 7.0 g catalase (Novozyme), and 0.1 g Antifoam 204 in 255 mL phosphate buffer (pH 7.0, 20 mM) was charged to the reactor. The temperature was set at 25°C, and 3 N NaOH was added to adjust the pH to 7.4. Pure oxygen (4 psig, or 207 mmHg) was applied to the enzyme solution prior to substrate addition. The substrate solution was prepared by dissolving 50 g NaHSO3 (0.45 mol) and 40 g 6 (0.36 mol) in 240 mL water. The substrate solution was charged to the enzyme solution over 19 h while maintaining the pH at 7.4 by titration of 3 N NaOH. By the end of the addition, 56.5 g of 3 N NaOH (0.15 mol) had been added to the reaction mixture, and conversion was 96.4%. For cyanation, CPME (240 mL) was added to the stream (compound 5 and 7) from enzymatic step. The solution was chilled to 10°C. A solution of NaCN in 70 mL water (22.9 g, 0.45 mol) was added over 0.5 h. The cyanation reached > 98% conversion after additional 0.5 h at 10°C. Significant protein precipitation took place when cyanide was added. The insoluble was removed by filtration through celite. After phase split, the aqueous phase was back extracted with 100 mL CPME. In methanolysis step, the CPME solution of 8 was added to HCl in MeOH (260 mL, [HCl] ≥ 6 N) at 5-10°C over 1 h. The solution was heated to 50°C and agitated for 4 h to complete the conversion. For product isolation, the volume was reduced to 160 mL by evaporation, followed by extraction under basic condition to give a solution of 9 in MTBE (375 mL). In the final step, the product was isolated by crystallization after forming the HCl salt. The MTBE solution was added to HCl in a mixture of 60 mL iPrOH and 240 mL MTBE at 0°C. The solution was seeded to crystallize for 5 h at -10°C. After solid isolation and drying under vacuum, 41.2 g (0.20 mol, 56% overall yield) of 2 was obtained in >98.5% purity and >99.9% e.e. |
| | Boceprevir InterMediates Preparation Products And Raw materials |
|