- Cinchocaine
-

- $45.00 / 1kg
-
2024-04-30
- CAS:85-79-0
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 300tons
- Cinchocaine
-

- $3.00 / 1kg
-
2024-04-19
- CAS:85-79-0
- Min. Order: 1kg
- Purity: 99.9%
- Supply Ability: 10 tons
- Cinchocaine
-

- $25.00/ kg
-
2024-02-21
- CAS:85-79-0
- Min. Order: 1kg
- Purity: 99.8%
- Supply Ability: 200tons/year
|
| | Cinchocaine Basic information |
| Product Name: | Cinchocaine | | Synonyms: | 2-butoxy-n-(2-(diethylamino)ethyl)-4-quinolinecarboxamid;2-butoxy-n-(2-(diethylamino)ethyl)-cinchoninamid;2-butoxy-n-(2-(diethylamino)ethyl)cinchoninamide;2-butoxy-n-(2-(diethylamino)ethyl-4-quinolinecarboxamid;2-Butoxy-N-(beta-diethylaminoethyl)cinchoninamide;2-Butoxy-N-[2-(diethylamino)ethyl]cinchoninamide;2-Butoxyquinoline-4-carboxylic acid diethylaminoethylamide;2-butoxyquinoline-4-carboxylicaciddiethylaminoethylamide | | CAS: | 85-79-0 | | MF: | C20H29N3O2 | | MW: | 343.46 | | EINECS: | 201-632-1 | | Product Categories: | research chemical;DIA - DICDrugs of Abuse;Alphabetic;Chemical Structure;D;Others;85-79-0 | | Mol File: | 85-79-0.mol | 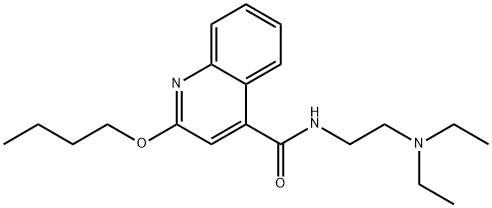 |
| | Cinchocaine Chemical Properties |
| Melting point | 64° | | Boiling point | 478.73°C (rough estimate) | | density | 1.1145 (rough estimate) | | refractive index | 1.6300 (estimate) | | storage temp. | Store at -20°C | | solubility | DMSO:45.0(Max Conc. mg/mL);131.02(Max Conc. mM) | | pka | pKa -5.3(aq. H2SO4) (Uncertain) | | form | Solid:particulate/powder | | Water Solubility | 68.01mg/L(temperature not stated) | | InChI | InChI=1S/C20H29N3O2/c1-4-7-14-25-19-15-17(16-10-8-9-11-18(16)22-19)20(24)21-12-13-23(5-2)6-3/h8-11,15H,4-7,12-14H2,1-3H3,(H,21,24) | | InChIKey | PUFQVTATUTYEAL-UHFFFAOYSA-N | | SMILES | N1C2C(=CC=CC=2)C(C(NCCN(CC)CC)=O)=CC=1OCCCC | | LogP | 4.4 at 25℃ and pH7 | | CAS DataBase Reference | 85-79-0(CAS DataBase Reference) | | NIST Chemistry Reference | Dibucaine(85-79-0) |
| | Cinchocaine Usage And Synthesis |
| Originator | Cincain,Ophtha | | Uses | Cinchocaine is a local anesthesic,used for Na+ channel blocker. | | Definition | ChEBI: A monocarboxylic acid amide that is the 2-(diethylamino)ethyl amide of 2-butoxyquinoline-4-carboxylic acid. One of the most potent and toxic of the long-acting local anesthetics, its parenteral use was restricted to spinal anesthesia. It is now generally o
ly used (usually as the hydrochloride) in creams and ointments and in suppositories for temporary relief of pain and itching associated with skin and anorectal conditions. | | Manufacturing Process | A benzene solution of 2.2 parts of α-chloro-γ-quinoline-carboxylic acid chloride
is gradually mixed, while cooling, with 2.3 parts of unsymmetrical
diethylethylenediamine. When the reaction is at an end the solution is washed
with water and the new base extracted by means of hydrochloric acid. The
base is precipitated by means of sodium carbonate and extracted with
benzene. The solvent is distilled and the base recrystallized from petroleum
ether. The α-chloro-γ-quinoline-carboxylic acid diethyl-amino-ethylene amide
forms colorless lamina crystals of melting point 74°C. With acids the base
forms neutral salts soluble in water.
A solution of 2.5 parts of sodium in n-butylalcohol is boiled with 30 parts of α-
chloro-γ-quinoline-carboxylic acid diethyl-amino-ethylene-amide in a reflux
apparatus, and when the reaction is over the excess of butylalcohol is
distilled. The remaining base is taken up with ether; the solution is washed
with water and dried. The solvent is then distilled. The α-n-butoxy-γ-quinoline�carboxylic acid diethyl-amino-ethylene-amide forms as colorless crystals, after
recrystallization from petroleum ether melting point of it 64°C.
In practice it is usually used as hydrochloride. | | Brand name | Nupercaine (Novartis). | | Therapeutic Function | Local anesthetic | | General Description | Articaine has a secondary nitrogen with a pKa of 7.8. It containsan aromatic thiophene ring bioisostere of the phenylring found in most other amide anesthetics. The log P of abenzene ring is 2.13 and the thiophene ring log P is 1.81,thus the thiophene ring is more hydrophilic than a phenylring. Although the thiophene ring has less lipid solubilitythan a phenyl ring, articaine is a lipid-soluble compound dueto the propylamine, the branched methyl and the substitutionson the thiophene ring. The onset of action of articaineis similar to lidocaine’s onset of action. | | Contact allergens | Dibucaine hydrochloride is an amide group local anesthetic
that can induce allergic contact dermatitis. | | Clinical Use | Articaine is availablein a 4% solution with epinephrine for use in infiltrationand nerve block anesthesia.Articaine is metabolized rapidly via plasma and tissuecarboxyesterase to its primary metabolite, the inactive,water-soluble carboxylic acid. Approximately 40% to 70%of articaine administered epidurally is metabolized to thecarboxylic acid, articainic acid. Approximately 4% to 15%of the articainic acid undergoes glucuronide conjugationand only 3% of the dose is recovered unchanged in theurine. The rapid plasma metabolism and reported inactivityof the carboxylic acid metabolite make articaine a potentiallysafer anesthetic agent when multiple or large dosesare necessary. |
| | Cinchocaine Preparation Products And Raw materials |
|