PF-04965842(Abrocitinib) manufacturers
- Abrocitinib
-

- $0.00 / 1g
-
2024-03-12
- CAS:1622902-68-4
- Min. Order: 1g
- Purity: 98% HPLC
- Supply Ability: 1kg
- Abrocitinib
-

- $0.00 / 1kg
-
2023-11-01
- CAS:1622902-68-4
- Min. Order: 1kg
- Purity: 99.0%
- Supply Ability: 20 tons
|
| | PF-04965842(Abrocitinib) Basic information |
| Product Name: | PF-04965842(Abrocitinib) | | Synonyms: | N-((1s,3s)-3-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)cyclobutyl)propane-1-sulfonamide;1-Propanesulfonamide, N-[cis-3-(methyl-7H-pyrrolo[2,3-d]pyrimidin-4-ylamino)cyclobutyl]-;PF-04965842 >=98% (HPLC);PF-04965842;Abrocitinib;rel-N-((1s,3s)-3-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)cyclobutyl)propane-1-sulfonamide;PF-04965842;PF 04965842;PF04965842;N-[cis-3-(Methyl-7H-pyrrolo[2,3-d]pyrimidin-4-ylamino)cyclobutyl]-1-propanesulfonamide | | CAS: | 1622902-68-4 | | MF: | C14H21N5O2S | | MW: | 323.41 | | EINECS: | | | Product Categories: | APIS;api | | Mol File: | 1622902-68-4.mol | 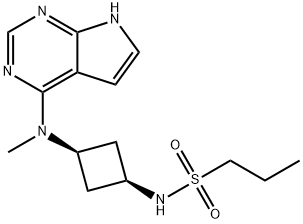 |
| | PF-04965842(Abrocitinib) Chemical Properties |
| density | 1.36±0.1 g/cm3(Predicted) | | storage temp. | room temp | | solubility | DMSO:100.0(Max Conc. mg/mL);309.2(Max Conc. mM) | | pka | 10.55±0.40(Predicted) | | form | powder | | color | white to beige |
| | PF-04965842(Abrocitinib) Usage And Synthesis |
| Characteristics | Class: non-receptor tyrosine kinase
Treatment: atopic dermatitis
Oral bioavailability = 60%
Elimination half-life = 5 h
Protein binding = 50% | | Biochem/physiol Actions | PF-04965842 is a Janus Kinase (JAK) inhibitor selective for JAK1 with an IC50 value of 29 nM for JAK1 compared to 803 nM for JAK2, >10000 nM for JAK3 and 1250 nM for Tyk2. JAKs mediate cytokine signaling, and are involved in cell proliferation and differentiation. PF-04965842 has been investigated as a possible treatment for psoriasis. | | Mechanism of action |
PF-04965842(Abrocitinib) is an orally bioavailable, selective JAK1 inhibitor to treat moderate-to-severe atopic dermatitis. Abrocitinib preferentially blocks cytokine signaling involving JAK1 and is selective against signaling pathways using dual JAK2 or JAK2/TYK2.
| | Clinical Use |
PF-04965842(Abrocitinib), a selective JAK1 inhibitor, was approved in 2022 for the treatment of adults with refractory, moderate-to-severe atopic dermatitis (AD) whose disease is not adequately controlled with other systemic drug products or when the use of those therapies is inadvisable.
| | Metabolism | The oral absorption of abrocotinib is 91%, but its oral bioavailability is moderate (60%) due to hepatic metabolism.3,4 Human PK studies with oral administration of [14C]-abrocitinib showed that the parent drug was the most abundant circulating species (26%), along with 3 oxidative metabolites: PF-06471658 (11%), PF-07055087 (12%), and PF- 07054874 (14%). The two hydroxyl compounds are active metabolites, with comparable JAK1 selectivity profiles to abrocitinib, whereas the pyrrolidinone pyrimidine metabolite lacks in kinase activity. Based on the in vitro cytochrome P450 phenotyping studies in human hepatocytes, CYP2C19 and CYP2C9 are the major CYP isoforms involved in the oxidative metabolism of abrocitinib, contributing to 53% and 30% of overall metabolism, respectively. As abrocotinib is primarily cleared in the liver, impairment in hepatocellular function may affect its pharmacokinetic properties, which could potentially impact its safety and/or efficacy. Abrocitinib is quite lipophilic with logD (pH 7.4) value of 1.9, which contributes to a modest plasma protein binding of 64% in humans. Its terminal half-life of 5 h along with 60% oral bioavailability appears to be adequate for once-daily, oral treatment of moderate-to-severe atopic dermatitis. |
| | PF-04965842(Abrocitinib) Preparation Products And Raw materials |
|