- Plx-4032 (RG7024)
-

- $15.00 / 1KG
-
2021-07-02
- CAS:1029872-54-5
- Min. Order: 1KG
- Purity: 99%+ HPLC
- Supply Ability: Monthly supply of 1 ton
- Vemurafenib
-

- $0.00 / 1KG
-
2020-04-30
- CAS:1029872-54-5
- Min. Order: 1KG
- Purity: 99.0%+
- Supply Ability: 800 tons
|
| | Plx-4032 (RG7024) Basic information |
| Product Name: | Plx-4032 (RG7024) | | Synonyms: | Plx-4032 (RG7024);PLX 4032; RG 7204; RO 5185426;RG 7204;RO 5185426;N-[3-[[5-(4-Chlorophenyl)-1H-pyrrolo[2,3-b]pyridin-3-yl]carbonyl]-2,4-difluorophenyl]-1-PropanesulfonaMide;N-(3-{[5-(4-chlorophenyl)-1H-pyrrolo[2,3-b]pyridin-3-yl]carbonyl}-2,4-difluorophenyl)propane-1-sulfonaMide;VeMurafenib,PLX 4032;1-PropanesulfonaMide, N-[3-[[5-(4-chlorophenyl)-1H-pyrrolo[2,3-b]pyridin-3-yl]carbonyl]-2,4-difluorophenyl]- | | CAS: | 1029872-54-5 | | MF: | C23H18ClF2N3O3S | | MW: | 489.9221264 | | EINECS: | 800-227-2 | | Product Categories: | | | Mol File: | 1029872-54-5.mol | 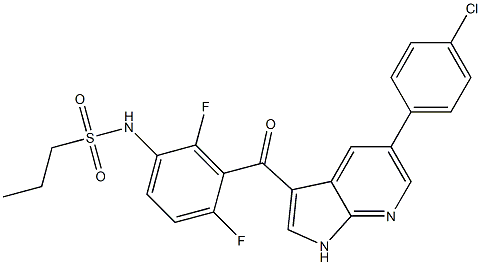 |
| | Plx-4032 (RG7024) Chemical Properties |
| Melting point | >263oC (dec.) | | storage temp. | Refrigerator | | solubility | DMSO (Slightly, Heated), Methanol (Slightly, Heated) | | form | Solid | | color | Off-White | | CAS DataBase Reference | 1029872-54-5 |
| | Plx-4032 (RG7024) Usage And Synthesis |
| Uses | Vemurafenib selective BRAFV600E kinase inhibitor; an antitumor agent. Vemurafenib functions by inhibiting the proliferation and mitogen-activated protein/extracellular signal-regulated kinase (ERK) kinase and ERK phosphorylation in a panel of tumor cell lines, including melanoma cell lines expressing BRAFV600E or other mutant BRAF proteins altered at codon 600. Potent B-Raf inhibitor | | Definition | ChEBI: Vemurafenib is a pyrrolopyridine that is 1H-pyrrolo[2,3-b]pyridine which is substituted at position 5 by a p-chlorophenyl group and at positions 3 by a 3-amino-2,6-difluorobenzoyl group, the amino group of which has undergone formal condensation with propane-1-sulfonic acid to give the corresponding sulfonamide. An inhibitor of BRAF and other kinases. It has a role as an antineoplastic agent and a B-Raf inhibitor. It is a pyrrolopyridine, a sulfonamide, a member of monochlorobenzenes, a difluorobenzene and an aromatic ketone. | | Clinical Use | Vemurafenib was originally discovered at Plexxikon and has
been co-developed by Roche and Plexxikon as an oral BRAF
inhibitor for the treatment of patients with BRAFV600E mutation-
positive metastatic melanoma. The drug displays good potency
and selectivity for the V600E mutation (IC50 = 3.2–14 nM), an
oncoprotein, over the wild-type BRAF (IC50 = 21–370 nM). The
compound is less potent in in vitro kinase assays than other Plexxikon
BRAF inhibitors, but it was selected for clinical development based on its enhanced potency against the BARFV600E-containing
A374 melanoma cell line. | | Synthesis | The synthesis described below is
based on a recent process patent (the Scheme). 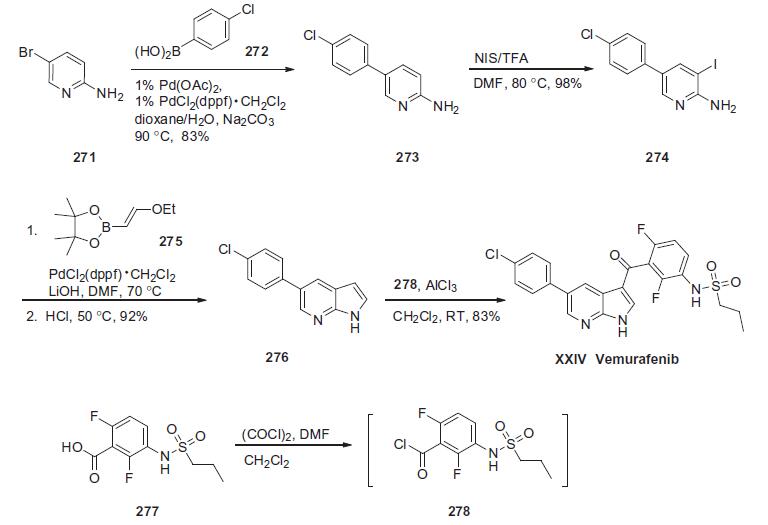
Commercially available 2-amino-5-bromopyridine (271) was
treated with 4-chlorophenylboronic acid (272) in the presence of
Na2CO3 and a catalytic amount of Pd(OAc)2/PdCl2(dppf)�CH2Cl2 to
give Suzuki product 273 in 83% yield. Arene 273 was subjected
to iodination conditions using NIS and TFA to provide iodide 274
in 98% yield. Iodide 274 and pinacol vinylboronate 275 were coupled
under Suzuki conditions followed by treatment with acid to
affect a tandem coupling¨Ccyclization sequence which resulted in
pyrimidyl pyrrole 276 in good yield. This material was treated with
aluminum trichloride and then subjected to the the acyl chloride of
commercially available sulfonamide acid 277, triggering a Friedel-
Crafts reaction providing vemurafenib (XXIV) in 85% yield. | | Drug interactions | Potentially hazardous interactions with other drugs
Anticoagulants: possibly enhances anticoagulant
effect of warfarin.
Antipsychotics: avoid concomitant use with
clozapine, risk of agranulocytosis.
Oestrogens and progestogens: contraceptive effect
possibly reduced. | | Metabolism | Only 5
% of a dose of vemurafenib is metabolised. 94
% of the dose is excreted in the faeces and 1
% in the urine. |
| | Plx-4032 (RG7024) Preparation Products And Raw materials |
|