- Cefpirome
-

- $0.00 / 25Kg/Drum
-
2021-10-21
- CAS:84957-29-9
- Min. Order: 1KG
- Purity: 98%min
- Supply Ability: 1000kg
- Cefpirome USP/EP/BP
-
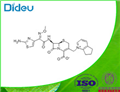
- $1.10 / 1g
-
2021-07-01
- CAS:84957-29-9
- Min. Order: 1g
- Purity: 99.9%
- Supply Ability: 100 Tons Min
- Cefpirome
-

- $100.00 / 1Kg/Bag
-
2020-11-16
- CAS:84957-29-9
- Min. Order: 1Kg/Bag
- Purity: 99
- Supply Ability: 999
|
| | Cefpirome Basic information |
| Product Name: | Cefpirome | | Synonyms: | CEFPIROME;1-[[(6R,7R)-7-[2-(2-amino-4-thiazolyl)glyoxylamido]-2-carboxy-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-yl]methyl]-6,7-dihydro-5H-1-pyrindinium hydroxide, inner salt, 72-(Z)-(O-methyloxime);3-[(2,3-cyclopenteno-1-pyridinium)methyl]-7-[2-syn-methoxyimino-2-(2-aminothiazol-4-yl)acetamido]ceph-3-em-4-carboxylate;(6R,7R)-7-(()-2-(2-Amino-4-thiazolyl)-2-methoxyiminoacetamido)-8-oxo-3-(2beta-trimethylenpyridinio)methyl)-5-thia-1-azabicyclo(4.2.0)oct-2-en-2-carboxylat;1-(((6R,7R)-7-(2-(2-Amino-4-thiazolyl)glyoxylamino)-2-carboxy-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-en-3-yl)methyl)-6,7-dihydro-5H-1-pyridinium hydroxide, inner salt;5H-1-Pyrindinium, 1-((7-(((2-amino-4-thiazolyl)(methoxyimino)acetyl)amino)-2-carboxy-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-en-3-yl)methyl)-5,6,7,8-tetrahydro-, hydroxide, inner salt, (6R-(6alpha,7beta(Z)))-;5H-1-Pyrindinium, 1-((7-(((2-amino-4-thiazolyl)(methoxyimino)acetyl)amino)-2-carboxy-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-en-3-yl)methyl)-6,7-dihydro-, hydroxide, inner salt, (6R-(6alpha,7beta(Z)))-;Cefpiroma | | CAS: | 84957-29-9 | | MF: | C22H22N6O5S2 | | MW: | 514.58 | | EINECS: | 1592732-453-0 | | Product Categories: | Cefpirome;Pharmaceutical intermediate | | Mol File: | 84957-29-9.mol | 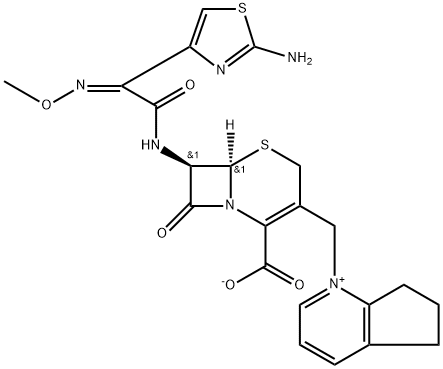 |
| | Cefpirome Chemical Properties |
| storage temp. | 2-8°C(protect from light) |
| Hazard Codes | Xi | | Risk Statements | 36/37/38-42/43 | | Safety Statements | 22-26-36/37/39 | | Toxicity | LD50 in mice, rats (g/kg): 1.9-2.4, 1.9-2.15 i.v.; 3.8-4.2, 5.8-6.55 i.p. (Donaubauer, Mayer) |
| | Cefpirome Usage And Synthesis |
| Description | This drug is resistant with respect to a broad spectrum of beta-lactamases. Its spectrum of
activity is analogous to that of the third-generation cephalosporin cefotaxime (32.1.2.56),
although it is more active with respect to some staphylococci, enterococci, and also a few
enterobacteria. Synonyms of this drug are cefrom, cedixen, and others. | | Uses | Cephalosporin antibiotic Claforan | | Definition | ChEBI: Cefpirome is a fourth-generation cephalosporin antibiotic having 6,7-dihydro-5H-cyclopenta[b]pyridinium-1-ylmethyl and [(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-(methoxyimino)acetyl]amino side groups located at positions 3 and 7 respectively. It is a cyclopentapyridine and a cephalosporin. | | Brand name | Cefrom (Hoechst-Roussel). | | Antimicrobial activity | A semisynthetic aminothiazoyl cephalosporin formulated as
the sulfate for parenteral administration. Activity against common
pathogens is similar to that of cefotaxime
and ceftriaxone, but it is more active against Ps. aeruginosa.
Unlike other cephalosporins it exhibits activity against some
strains of enterococci (MIC 4–16 mg/L), but this is of doubtful
clinical benefit. It is generally very stable to β-lactamases.
It is active against strains of Enterobacter spp., Citrobacter spp.,
Hafnia spp., Providencia spp., Ser. marcescens and Pr. vulgaris
producing molecular class C cephalosporinases.
Sten. maltophilia is resistant.
A 1 g intramuscular injection produces a plasma concentration
of 25 mg/L after 1.6–2.3 h. A similar intravenous
dose achieves a peak concentration of 97 mg/L. The plasma
half-
life is 1.4–2.3 h and protein binding is around 10%. It
is well distributed, achieving therapeutic concentrations in
tissues and exudates. It penetrates poorly into CSF in the
absence of meningeal inflammation, but concentrations
around 2–4 mg/L have been found in patients with purulent
meningitis.
Little, if any, of the drug is metabolized and most is excreted
unchanged in the urine within 12 h, mainly by glomerular filtration.
Clearance declines in proportion to renal function.
Around 60% of the drug is removed in 3 h by hemodialysis.
Low concentrations are found in breast milk.
Side effects are those common to other cephalosporins.
Diarrhea is common and occasional cases of pseudomembranous
colitis have been reported.
It is mainly used in the treatment of serious sepsis, particularly
nosocomial infections in which resistant Gram-negative
pathogens are known or suspected to be involved. It is not
widely available, but is marketed in Japan. | | Clinical Use | Cefpirome (Cefrom) is a newer parenteral, -lactamase–resistant cephalosporin with a quaternary ammonium groupat the 3-position of the cephem nucleus. Because its potencyagainst Gram-positive and Gram-negative bacteria rivals thatof the first-generation and third-generation cephalosporins,respectively, cefpirome is being touted as the first fourthgenerationcephalosporin. Its broad spectrum includesmethicillin-sensitive staphylococci, penicillin-resistantpneumococci, and β-lactamase–producing strains of E. coli,Enterobacter, Citrobacter, and Serratia spp. Its efficacyagainst P. aeruginosa is comparable with that of ceftazidime.Cefpirome is excreted largely unchanged in the urine with ahalf-life of 2 hours. | | Synthesis | Cefpirome, {6R-[6α,7β(Z)]}-1-[(7-{[(2-amino-4-thiazolyl)-(methoximino)
acetyl]amino}-2-carboxy-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-yl)methyl-
1-methyl]pyrrolidine chloride (32.1.2.100), is also synthesized by methods described for syn�thesizing third-generation cephalosporins, in particular, ceftazidime (32.1.2.82). |
| | Cefpirome Preparation Products And Raw materials |
|