- Gatifloxacin
-
- $0.00 / 25kg
-
2024-04-26
- CAS:112811-59-3
- Min. Order: 25kg
- Purity: 99.0%
- Supply Ability: 10tons
- Gatifloxacin
-

- $0.00 / 1KG
-
2024-03-16
- CAS:112811-59-3
- Min. Order: 100g
- Purity: 98%+
- Supply Ability: 100kg
- Gatifloxacin
-

- $0.00 / 1kg
-
2023-09-26
- CAS:112811-59-3
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20tons
Related articles - Toxicity of Gatifloxacin
- Gatifloxacin is an 8-methoxy fluoroquinolone and is marketed by Bristol Myers Squibb as Tequint. Three formulations are availa....
- Mar 18,2022
|
| | Gatifloxacin Basic information |
| | Gatifloxacin Chemical Properties |
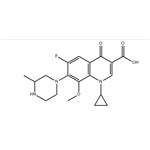
| Melting point | 162°C | | Boiling point | 607.8±55.0 °C(Predicted) | | density | 1.386±0.06 g/cm3(Predicted) | | storage temp. | Sealed in dry,2-8°C | | solubility | Chloroform (Slightly, Heated), Methanol (Slightly, Heated) | | pka | 6.43±0.50(Predicted) | | form | White to yellow crystalline powder. | | color | White to Off-White | | InChI | InChI=1S/C19H22FN3O4/c1-10-8-22(6-5-21-10)16-14(20)7-12-15(18(16)27-2)23(11-3-4-11)9-13(17(12)24)19(25)26/h7,9-11,21H,3-6,8H2,1-2H3,(H,25,26) | | InChIKey | XUBOMFCQGDBHNK-UHFFFAOYSA-N | | SMILES | N1(C2CC2)C2=C(C=C(F)C(N3CCNC(C)C3)=C2OC)C(=O)C(C(O)=O)=C1 | | CAS DataBase Reference | 112811-59-3(CAS DataBase Reference) |
| Hazard Codes | Xn | | Risk Statements | 20/21/22 | | Safety Statements | 36/37 | | HS Code | 29335990 |
| | Gatifloxacin Usage And Synthesis |
| Description | Gatifloxacin belongs to a class of drugs known as quinolone antibiotics and is used to treat acute sinus, lung, or urinary tract infections and sexually transmitted bacterial infection.This drug may be taken orally, in tablet form, or by injection.Common side effects associated with gatifloxacin include nausea, vaginitis(irritation or inflammation of the vagina),diarrhea, headache, dizziness, and irregular heart beats. In general, gatifloxacin is used in people who are unresponsive to other AOM therapies.
In one study, gatifloxacin was compared with amoxicillin/clavulanate in the treatment of recurrent otitis media (OM) and AOM in treatment failures in children.Three hundred fifty-four infants and children with recurrent OM or AOM failure received gatifloxacin or amoxicillin/clavulanate.Results showed that both drugs were well tolerated; the most common side effect was diarrhea.Researchers concluded that treatment with gatifloxacin once daily was as effective as amoxicillin/clavulanate twice daily.In other medical literature, gatifloxacin has been noted as a third-line treatment option in AOM. | | Chemical Properties | White to light yellow powder | | Uses | An antibacterial. | | Definition | ChEBI: A monocarboxylic acid that is 4-oxo-1,4-dihydroquinoline-3-carboxylic acid which is substituted on the nitrogen by a cyclopropyl group and at positions 6, 7, and 8 by fluoro, 3-methylpiperazin-1-yl, and methoxy groups, respectively. Gatifloxacin is an anti
iotic of the fourth-generation fluoroquinolone family, that like other members of that family, inhibits the bacterial topoisomerase type-II enzymes. | | Brand name | Tequin (Bristol-Myers Squibb); Zymar (Allergan). | | Antimicrobial activity | Gatifloxacin is an 8-methoxyfluoroquinolone with enhanced activity against Gram-positive, atypical agents, and some anerobes, and broad-spectrum activity against Gram-negative bacteria. It is bactericidal and produces a post-antibiotic effect in Gram-positive and Gramnegative bacteria.
The in vitro antibacterial spectrum of gatifloxacin has been tested against a variety of clinically important microorganisms. It is two to four times more potent than ciprofloxacin and ofloxacin against staphylococci, streptococci, pneumococci, and enterococci. However, it is two times less potent than ciprofloxacin, but the same as or two times more potent than ofloxacin against Enterobacteriaceae. Gatifloxacin and ofloxacin have similar antipseudomonal activity, while ciprofloxacin is two to eight times more potent. Gatifloxacin is highly potent against Hemophilus influenzae, Legionella species, and Helicobacter pylori, and also has activity against Bacteroides fragilis and Clostridium difficile. Like other quinolones, it has poor activity against Mycobacterium avium intracellulare, but is 8–16 times more potent against Mycobacterium tuberculosis.
Meyler's Side Effects of Drugs || Gatifloxacin | | Pharmaceutical Applications | The spectrum includes Acinetobacter spp. and Aeromonas spp. but it is not very active against Ps. aeruginosa and other non-fermentative Gram-negative rods. It is more active against methicillin-susceptible strains of staphylococci than methicillin-resistant strains. It is also active against Chlamydia, Mycoplasma and Legionella spp. and has some activity against anaerobes.
It is almost completely absorbed when given orally and is widely distributed throughout the body into many body tissues and fluids. The plasma half-life is 6–8 h. More than 70% of the drug is excreted unchanged in the urine. Renal clearance is reduced by 57% in moderate renal insufficiency and by 77% in severe renal insufficiency.
Prolongation of the QTc interval in some patients and interference with diabetes mellitus have resulted in withdrawal of the drug in most countries for systemic usage. Gatifloxacin remains in use in North America only as an ophthalmic solution. | | Biological Activity | Fluoroquinolone antibiotic. Inhibits bacterial type II topoisomerases (IC 50 values are 0.109 and 13.8 μ g/ml for E.coli DNA gyrase and S.aureus topoisomerase IV respectively). Displays potent activity against gram-positive and gram-negative bacteria. Stimulates short-term self-renewal in both human and mouse embryonic stem cells in vitro . | | Mechanism of action | Gatifloxacin is a quinolone antimicrobial. It is an 8-methoxyfluoroquinolone with a 3-methylpiperazinyl substituent at C7. The antibacterial action of gatifloxacin results from inhibition of DNA gyrase and topoisomerase IV. DNA gyrase is an essential enzyme that is involved in the replication, transcription, and repair of bacterial DNA. Topoisomerase IV is an enzyme known to play a key role in the partitioning of the chromosomal DNA during bacterial cell division. The mechanism of action of fluoroquinolones including gatifloxacin is different from that of aminoglycoside, macrolide, and tetracycline antibiotics. Therefore, gatifloxacin may be active against pathogens that are resistant to these antibiotics and these antibiotics may be active against pathogens that are resistant to gatifloxacin. There is no cross-resistance between gatifloxacin and the aforementioned classes of antibiotics. Cross-resistance has been observed between systemic gatifloxacin and some other fluoroquinolones.
https://www.accessdata.fda.gov | | Pharmacokinetics | Gatifloxacin ophthalmic solution 0.5% was administered to one eye of 6 healthy male subjects each in an escalated dosing regimen starting with a single 2 drop dose, then 2 drops 4 times daily for 7 days, and finally 2 drops 8 times daily for 3 days. At all time points, serum gatifloxacin levels were below the lower limit of quantification (5 ng/mL) in all subjects. | | Side effects | Gatifloxacin is well absorbed from the gastrointestinal tract (oral availability almost 100%), and concomitant administration of a continental breakfast, 1050 kcal, had no effect on its availability. The standard dose is 400 mg od and both oral and intravenous formulations are available.
Common side effects
Worsening of eye infection
Eye irritation
Eye pain
Change in taste
The following serious adverse reactions are described elsewhere in the labeling:
Hypersensitivity [see Contraindications (4) and Warnings and Precautions (5.1)]
Growth of Resistant Organisms With Prolonged Use [see Warnings and Precautions (5.2)]
Corneal Endothelial Cell Injury [see Warnings and Precautions (5.3)]
https://go.drugbank.com
https://medlineplus.gov
https://www.accessdata.fda.gov | | storage | +4°C |
| | Gatifloxacin Preparation Products And Raw materials |
|