| Company Name: |
kemikalieimport
|
| Tel: |
+ 45 - 2034 3359 |
| Email: |
Sales@kemikalieimport.dk |
| Products Intro: |
|
|
| | nifurzide Basic information |
| Product Name: | nifurzide | | Synonyms: | nifurzide;5-Nitro-2-thiophenecarboxylic acid N'-[3-(5-nitro-2-furyl)-2-propenylidene] hydrazide;N'-[3-(5-Nitro-2-furyl)-2-propenylidene]-5-nitro-2-thiophenecarbohydrazide;2-Thiophenecarboxylic acid, 5-nitro-, 2-[3-(5-nitro-2-furanyl)-2-propen-1-ylidene]hydrazide;Ricridene | | CAS: | 39978-42-2 | | MF: | C12H8N4O6S | | MW: | 336.28 | | EINECS: | 2547280 | | Product Categories: | | | Mol File: | 39978-42-2.mol | 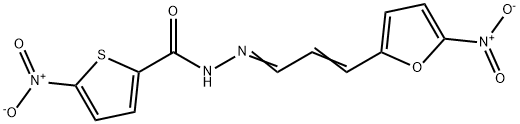 |
| | nifurzide Chemical Properties |
| Melting point | 235-236° | | density | 1.62±0.1 g/cm3(Predicted) | | pka | 10.31±0.46(Predicted) |
| Toxicity | LD50 orally in mice: 3200 mg/kg (Szarvasi, Fontaine, 1972) |
| | nifurzide Usage And Synthesis |
| Originator | Ricridene,Anphar,Switz.,1981 | | Definition | ChEBI: Nifurzide is a member of thiophenes and a C-nitro compound. | | Manufacturing Process | (a) Ethyl-5-nitro-2-thiophene carboxylate:
17.4 g (mol/10 = 17.31 g) of 5-nitrothiophene carboxylic acid are dissolved in
85 ml of absolute ethanol. A stream of gaseous hydrochloric acid is caused to
enter the boiling solution to the point of saturation, and for 5 hours.
Evaporation to dryness takes place and then the solid residue is washed with
a sodium bicarbonate solution. It is suction-filtered and washed with water.
After drying, there are obtained 17.7 g of a yellow product with a melting
point of 63°C to 65°C and the yield is 88% (theoretical yield = 88%).
The N'-(5'-nitro-2'-thenoyl)hydrazide is prepared by reacting hydrazine with
ethyl 5-nitro-2-thiophene carboxylate.
(b) 6.3 g (mol/30 = 6.5 g) of N1-[5'-nitro-2'-thenoyl]hydrazide are dissolved
in 100 ml of dry tetrahydrofuran. 5.6 g (mol/30 = 5.55 g) of 5-nitro-2-furyl
acrolein in 56 ml of tetrahydrofuran are added. Heating under reflux takes
place for 1 hour and, 25 minutes after starting the heating, the crystallization
commences; the crystals are suction-filtered, washed with ether and dried.
There are obtained 7.9 g (yield 70%-theoretical yield = 11.2 g) of a yellow
solid of melting point 235°C to 236°C.
Recrystallization (tepid dimethylformamide + ether) leaves the melting point
unchanged. | | Therapeutic Function | Antibacterial, Antidiarrheal |
| | nifurzide Preparation Products And Raw materials |
|