- Micronomicin
-

- $1.10 / 1g
-
2022-05-17
- CAS:52093-21-7
- Min. Order: 1g
- Purity: 99.9% Min
- Supply Ability: 100 Tons
- Micronomicin
-

- $95.00 / 1KG
-
2021-10-20
- CAS:52093-21-7
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 9000kg/per week
- Micronomicin
-

- $1.00 / 1g
-
2021-07-20
- CAS:52093-21-7
- Min. Order: 1g
- Purity: 99%
- Supply Ability: 50tons
|
| | Micronomicin Basic information |
| Product Name: | Micronomicin | | Synonyms: | Micronomicin;MICRONOMYCIN;Antibiotic KW 1062;Antibiotic XK 62-2;DE 020;D-Streptamine, O-2-amino-2,3,4,6-tetradeoxy-6-(methylamino)-a-D-erythro-hexopyranosyl-(14)-O-[3-deoxy-;Gentamicin C2b;KW 1062 | | CAS: | 52093-21-7 | | MF: | C20H41N5O7 | | MW: | 463.57 | | EINECS: | | | Product Categories: | | | Mol File: | 52093-21-7.mol | 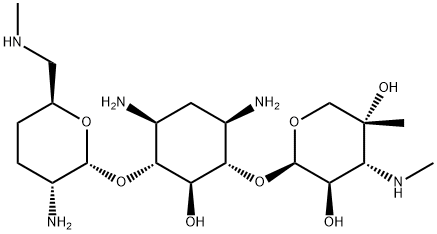 |
| | Micronomicin Chemical Properties |
| Melting point | 260° (dec) | | alpha | D20 +116° (c = 1 in water) | | Boiling point | 567.39°C (rough estimate) | | density | 1.2416 (rough estimate) | | refractive index | 1.7600 (estimate) | | storage temp. | 4°C, stored under nitrogen | | pka | 13.29±0.70(Predicted) | | Water Solubility | Water : 250 mg/mL (539.29 mM; Need ultrasonic) |
| Toxicity | LD50 i.v. in mice: 93 mg/kg (Okachi) |
| | Micronomicin Usage And Synthesis |
| Description | Micronomicin, formerly called sagamicin, was found in the culture broth of Micromonospora sagamiensis var. nonreducans by Kyowa Hakko Kogyo Company and Abbott Laboratories in 1974. The compound is identical with one of the minor components contained in gentamicin, gentamicin C2b, but it is produced as a single component. Its amino group at the 6 -position is methylated and is not subject to the enzymatic acetylation caused by resistant bacteria, including Pseudomonas aeruginosa. Micronomicin is less toxic than gentamicin to the renal and aural systems. | | Originator | Sagamicin,Kyowa Hakko,Japan,1982 | | Uses | Gentamicin C2b is a component of Gentamicin Sulphate Complex C. Gentamicin (G360600) is an antibiotic from bacteria Micromonospora purpurea and used to treat respiratory and urinary tract, blood, bone and soft tissues infections. | | Uses | Gentamicin C2b (micronomicin) is a trisaccharide pentaaminoglycoside antibiotic belonging to the gentamicin class. Gentamicin C2b was isolated from Micromonospora sagamiensis var. nonreductans by researchers at Kyowa Hakko Kogyo in 1974. Gentamicin C2b has broad activity against Gram positive and Gram negative bacteria. More recently, gentamicin C2b has been shown to induce neuromuscular blockage. | | Definition | ChEBI: Gentamicin C2b is an aminoglycoside. | | Manufacturing Process | A. Culturing of MK-65: In this example, Micromonospora sagamiensis MK-65
ATCC 21826 (FERM-P No. 1530) is used as the seed strain. One loopful of the
seed strain is inoculated into 30 ml of a first seed medium in a 250 ml�Erlenmeyer flask. The first seed medium has the following composition:
Percent
Dextrin 1
Glucose 1
Peptone 0.5
Yeast extract 0.5
CaCO3(pH: 7.2 before sterilization) 0.1
Culturing is carried out with shaking at 30°C for 5 days. 30 ml of the seed
culture is then inoculated into 300 ml of a second seed medium, of the same
composition as the first seed medium, in a 2 liter-Erlenmeyer flask provided
with baffles. The second seed culturing is carried out with shaking at 30°C for 2 days. Then 1.5 liters of the second seed culture (corresponding to the
content of 5 flasks) is inoculated into 15 liters of a third seed medium of the
same composition as set forth above, in a 30 liter-glass jar fermenter.
Culturing in the jar fermenter is carried out with aeration (15 liters/minute)
and stirring (350 rpm) at 30°C for 2 days. Then, 15 liters of the third seed
culture is inoculated into 60 liters of a fourth seed medium of the same
composition as set forth above, in a 300 liter-fermenter. Culturing in the
fermenter is carried out with aeration (60 liters/minute) and stirring (150
rpm) at 30°C for 2 days. Finally, 60 liters of the fourth seed culture is
inoculated into 600 liters of a fermentation medium having the following
composition in a 1,000 liter-fermenter.
Percent
Dextrin 5
Soybean meal 4
CaCO3 (pH: 7.2 before sterilization) 0.7
Culturing in the fermenter is carried out with aeration (600 liters/minute) and
stirring 150 rpm) at 35°C for 5 days.
B. Isolation of crude antibiotic: After the completion of fermentation, the
culture liquor is adjusted to a pH of 2.0 with 12 N sulfuric acid and stirred for
30 minutes. Then, about 10 kg of a filter aid, Radiolite No. 600 (product of
Showa Kagaku Kogyo Co., Ltd., Japan) is added thereto and the microbial cells
are removed by filtration. The filtrate is adjusted to a pH of 8.0 with 6N
sodium hydroxide and passed through a column packed with about 50 liters of
a cation exchange resin, Amberlite IRC-50 (ammonia form). The active
substance is adsorbed on the resin and the eluate is discarded. After washing
the resin with water, the active substance is eluted out with 1N aqueous
ammonia. The eluate is obtained in fractions and the activity of each of the
fractions is determined against Bacillus subtilis No. 10707 by a paper disk
method using an agar plate.
Active fractions are combined and concentrated in vacuo to about 5 liters. The
concentrate is then adjusted to a pH of 8.0 with 6N sulfuric acid and passed
through a column packed with 1 liter of an anion exchange resin, Dowex 1X2
(OH-form). The column is washed with about 5 liters of water and the effluent
and the washings containing active substance are combined and are
concentrated to 1/15 by volume. The concentrate is adjusted to a pH of 10.5
with 6 N sodium hydroxide and 5 volumes of acetone is added thereto. The
resultant precipitate is removed by filtration and the filtrate is concentrated to
500 ml. The concentrate is adjusted to a pH of 4.5 with 6 N sulfuric acid and
2.5 liters of methanol is added thereto. After cooling, a white precipitate is
obtained. The precipitate is separated by filtration and washed with methanol.
After drying in vacuo, about 300 g of white powder is obtained.
The white powder is a mixture of the sulfate of gentamicin C1a, and the
sulfate of XK-62-2, and exhibits an activity of 620 units/mg (the activity of 1
mg of pure product corresponds to 1,000 units).
C. Isolation and purification of XK-62-2: 100 g of the white powder obtained
in the above step B are placed to form a thin, uniform layer on the upper part
of a 5 cm x 150 cm column packed with about 3 kg of silica gel advancely
suspended in a solvent of chloroform, isopropanol and 17% aqueous ammonia(2:1:1 by volume). Thereafter, elution is carried out with the same solvent at
a flow rate of about 250 ml/hour. The eluate is separated in 100 ml portions.
The active fraction is subjected to paper chromatography to examine the
components eluted. XK-62-2 is eluted in fraction Nos. 53-75 and gentamicin
C1ais eluted in fraction Nos. 85-120. The fraction Nos. 53-75 are combined
and concentrated under reduced pressure to sufficiently remove the solvent.
The concentrate is then dissolved in a small amount of water. After freeze�drying the solution, about 38 g of a purified preparate of XK-62-2 (free base)
is obtained. The preparate has an activity of 950 units/mg. Likewise, fraction
Nos. 85-120 are combined and concentrated under reduced pressure to
sufficiently remove the solvent. The concentrate is then dissolved in a small
amount of water. After freeze-drying the solution, about 50 g of a purified
preparate of gentamicin C1a (free base) is obtained. The activity of the
preparate is about 980 units/mg. | | Therapeutic Function | Antibiotic | | Antimicrobial activity | Antibacterial and pharmacokinetic properties are similar to
those of its precursor gentamicin C1a but it is more resistant to
AAC(6′). Dosage is similar to that for gentamicin, and should
be controlled by blood level determinations. It is available in
Japan. |
| | Micronomicin Preparation Products And Raw materials |
|