|
ChemicalBook Optimization Suppliers |
|
| 化学名: | D-パントテン酸カルシウム | | 英語化学名: | Calcium D-Pantothenate | | 别名: | (+)-PANTOTHENIC ACID CALCIUM SALT;(R)-(+)-N-(2,4-DIHYDROXY-3,3-DIMETHYL-1-OXOBUTYL)-BETA-ALANINE HEMICALCIUM SALT;(theta)-;beta-alanine,n-(2,4-dihydroxy-3,3-dimethyl-1-oxobutyl)-,calciumsalt(2:1),;N-(2,4-dihydroxy-3,3-dimethyl-1-oxobutyl)-,calciumsalt(2:1),(R)-.beta.-Alanine;N-(2,4-Dihydroxy-3,3-dimethyl-1-oxobutyl)-alaninecalciumsalt;n-(2,4-dihydroxy-3,3-dimethylbutyryl)-beta-alaninecalcium;panthoject | | CAS番号: | 137-08-6 | | 分子式: | C9H17NO5.1/2Ca | | 分子量: | 476.53 | | EINECS: | 205-278-9 | | カテゴリ情報: | Aliphatics;Chiral Reagents;Intermediates & Fine Chemicals;Pharmaceuticals;Pyridines ,Halogenated Heterocycles;Food additives;Isolabel;Biochemistry;Ca (Calcium) Compounds;Classes of Metal Compounds;Typical Metal Compounds;Vitamins;Nutritional Supplements;Nutritional fortification substances | | Mol File: | 137-08-6.mol | 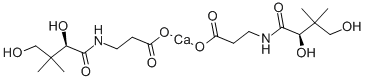 |
| 融点 | 190 °C | | 比旋光度 | 26.5 º (c=5, in water) | | 屈折率 | 27 ° (C=5, H2O) | | 闪点 | 145 °C | | 貯蔵温度 | 2-8°C | | 溶解性 | H2O: 50 mg/mL at 25 °C, clear, nearly colorless | | 外見 | Powder | | 色 | White or almost white | | PH | 6.8-7.2 (25℃, 50mg/mL in H2O) | | 臭い (Odor) | Odorless | | 光学活性 (optical activity) | [α]20/D +27±2°, c = 5% in H2O | | 水溶解度 | Soluble in water. | | Sensitive | Hygroscopic | | Merck | 14,7015 | | BRN | 3769272 | | 安定性: | Stable, but may be moisture or air sensitive. Incompatible with strong acids, strong bases. | | InChIKey | FAPWYRCQGJNNSJ-UBKPKTQASA-L | | LogP | -0.849 (est) | | CAS データベース | 137-08-6(CAS DataBase Reference) | | EPAの化学物質情報 | Calcium pantothenate (137-08-6) |
| | D-パントテン酸カルシウム Usage And Synthesis |
| 外観 | 白色の粉末 | | 性質 | 無色吸湿体液体.〔α〕D25 +37.5˚ (H2O).pK 4.4.中性溶液安定.酸・アルカリ,熱で速やかに分解.Ca塩は光,空気にかなり安定.水溶液の加熱滅菌不可 | | 定義 | 本品は、パントテン酸(*)のカルシウム塩であり、次の化学式で表される。
参照表示名称:パントテン酸 | | 溶解性 | 水に易溶, エタノールに難溶, エーテルに不溶。水に溶けやすく、エタノールに極めて溶けにくく、ジエチルエーテルにほとんど溶けない。 | | 解説 | パントテン酸カルシウム,水溶性ビタミンで,補酵素の構成成分である。生体内に取込まれると組織細胞中でコエンザイムA (CoA) に生合成される。動物実験では,欠乏の際に成長停止,毛髪,神経,消化管,副腎などの障害が認められる。腸内細菌により生成されるので,抗生物質投与により腸内細菌が死滅したような場合に欠乏症をきたす可能性がある。臨床的には成育期,妊娠時,病後回復期などに投与され,また,放射線療法,抗腫瘍剤,ストレプトマイシンなどの副作用防止に用いられる。副作用としては,1日 10~20gを内服で連続投与すると下痢,顔面浮腫などの症状を示すが,投薬を中止すれば消失する。 | | 生物学的性質 | 欠乏症:発育阻止(ネズミ,ニワトリ),皮膚障害(ネズミ),増殖阻止(酵母,乳酸菌) 機能:補酵素A,アシル運搬タンパク質成分 | | 用途 | 保健機能食品(栄養機能食品)
栄養機能食品は、栄養成分の機能の表示をして販売される食品です。栄養機能食品として販売するためには、一日当たりの摂取目安量に含まれる当該栄養成分量が定められた上?下限値の範囲内にある必要があるほか、栄養機能表示だけでなく注意喚起表示等も表示する必要があります。
栄養機能表示
パントテン酸は、皮膚や粘膜の健康維持を助ける栄養素です。
注意喚起表示
本品は、多量摂取により疾病が治癒したり、より健康が増進するものではありません。1日の摂取目安量を守ってください。
| | 用途 | ビタミン B 群化合物です。代
謝反応に作用します。 | | 用途 | 薬理研究用。生理作用研究用。 | | 化粧品の成分用途 | ヘアコンディショニング剤 | | 効能 | パントテン酸補充薬 | | 商品名 | パントテン酸カルシウム (丸石製薬) | | 使用上の注意 | 吸湿性あり | | 分布 | 広く分布 | | 化学的特性 | Calcium D-Pantothenate is white crystalline powder | | Originator | Calcium D-Pantothenate,Arocor Holdings Inc. | | 来歴 | Pantothenic acid (PA), also known as vitamin B5, is essential to all forms of life. Its name is derived from the Greek word pantos that means “everywhere”, which is appropriate for this widely, distributed vitamin. | | 使用 | D-(+)-Pantothenic acid calcium salt is a member of the B complex vitamins; essential vitamin for the biosynthesis of coenzyme A in mammalian cells. Occurs ubiquitously in all animal and plant tissue. The richest common source is liver, but jelly of the queen bee contains 6 times as much as liver. Rice bran and molasses are other good sources. | | 使用 | Calcium Pantothenate is a nutrient and dietary supplement which is the calcium chloride double salt of . It is a white powder of bitter taste and has a solubility of 1 g in 3 ml of water. It is used in special dietary foods. | | 使用 | calcium pantothenate is used as an emollient and to enrich creams and lotions in hair care preparations. This is the calcium salt of pantothenic acid found in liver, rice, bran, and molasses. It is also found in large amounts in royal jelly. | | 定義 | Calcium pantothenate, also known as calcium D-pantothenate or calcium pantothenic acid, is a naturally occurring form of pantothenic acid, an essential vitamin found in various foods. Calcium pantothenate is generally recognized as safe (GRAS) in the United States and in Europe. The US FDA requires infant formulas have at least 300 micrograms (mcg) per 100 calories of prepared formula. | | 定義 | ChEBI: Calcium pantothenate is a polymer. | | 反応性 | Pantothenic acid is a constituent of coenzyme A, which participates in numerous enzyme reactions. CoA was discovered as an essential cofactor for the acetylation of sulfanilamide in the liver and of choline in the brain. | | Manufacturing Process | A mixture of 288 g (4 mols) of isobutyraldehyde, 288 g of methanol was
cooled to 10°C and 170 g (2 mols) of 36.6% formalin containing 8.5 g (3%
based on isobutyraldehyde) of sodium hydroxide was added dropwise over a
55 minute period to produce alpha,alpha-dimethyl-beta-hydroxy-propionaldehyde. The mixture was stirred for an additional 2 hours at 10-15°C
and then contacted with acetic acid to neutralize the catalyst. The excess
isobutyraldehyde and methanol were stripped off at a kettle temperature of
50°C at 25 mm. To the residual α,α-dimethyl-beta-hydroxypropionaldehyde a
mixture of 260 ml of methanol and 2 g (0.75%) sodium cyanide was added
and the solution cooled to 10°C before adding 59.4 g (2.2 mols) of hydrogen
cyanide dropwise over a 35 minute period to produce α,γ-dihydroxy-β,β-
dimethylbutyronitrile. The mixture was stirred at 10°C for one hour period and
then contacted with acetic acid to neutralize the catalyst before stripping off
the excess methanol to a kettle temperature of 45°C at 18 mm. The crude
cyanohydrin was then hydrolysed by heating with 4 mols of concentrated
hydrochloric acid at 80°C for 2 hours, then diluting with an equal volume of
water and heating at 100°C for an additional 8 hours. The aqueous mixture
was extracted continuously with ethylene dichloride. The solvent was removed, and pantolactone (B. P. 131°C/19 mm, M.P. 61-77°C, 96.5% purity
by saponification) was obtained by distillation in 71.5% yield based on
formaldehyde and 55% efficiency based on isobutyraldehyde.
26 grams of racemic pantolactone (0.2 mol) and 1.1 grams of sodium
methoxide (0.02 mol) contained in 30 ml of methanol, were added to 78.8
grams of 1-brucine (0.2 mol) contained in 156 ml of methanol. The resulting
mixture was refluxed for 1.5 hours and allowed to stand at room temperature
overnight. After centrifuging, washing with methanol and drying, 65.4 grams
of D-(-)-pantolactone 1-brucine (62% of theory based upon all of the racemic
pantolactone) melting at 203° to 206°C were obtained. Upon chilling the
mother liquor, 13.46 grams of additional complex melting at 175° to 177°C
were obtained.
D-(-)-Pantolactone was obtained from the complex in the following manner.
The 65.4 grams of complex obtained above were treated with 65 ml of
chloroform and 5.35 grams of sodium hydroxide contained in 35 ml of water
for one hour at room temperature. The aqueous layer was extracted 6 times
with 20 ml portions of chloroform in order to remove the brucine. The sodium
pantoate contained in the aqueous layer was relactonized by treatment with
11 ml of concentrated hydrochloric acid. Extraction of the crude D-(-)-
pantolactone yielded 15.29 grams. This material was then recrystallized from
7 ml of methyl isobutyl ketone and 7 ml hexane thereby yielding 9.77 grams
of D-(-)-pantolactone (37% of theory). The αD25 was -44.8°.
Into a vessel equipped with an agitator and reflux condenser are placed
approximately 52 parts by weight of α-hydroxy-β,β-di-methyl-γ-butyrolactone,
approximately 36 parts by weight of β-alanine, about 40 parts by weight of
diethylamine and about 100 parts by weight of anhydrous methanol. The
mixture is stirred and refluxed for about 12 hours until the reaction is
complete as evidenced by the dissolution of the β-alanine. To this resulting
mass is gradually added 8 parts by weight of calcium metal nodules or pellets
and refluxing continued until the metal is dissolved. The diethylamine and
alcohol are distilled off until the residue becomes viscous. The viscous residue
is dried under vacuum at 100°C. The solid residue recovered, as biologically
assayed, indicated a 91% yield of calcium pantothenate. | | brand name | Calpan (BASF); Pantholin
(Lilly). | | Therapeutic Function | Vitamin | | 生物学の機能 | Calcium D-Pantothenate accelerates the wound healing process by increasing the number of migrating cells, their distance and hence their speed. In addition, cell division is increased and the protein synthesis changed. Hence, higher quantities of pantothenate are locally required to enhance wound healing.
| | 一般的な説明 | The first suggestion for the existance of vitamin B5 came from Carter et al. in 1930; although it was never characterized or isolated. This vitamin is synthesized by most green plants and microorganisms. Excellent sources of the vitamin are liver, egg yolk, whole grains, and fortified ready-to-eat cereals. However, as the original name implies, many foods contain sufficient pantothenic acid to supply dietary needs.
Chemically, pantothenic acid is considered to be aβ-alanine derivative of the asymmetric pantoic acid and thus shows asymmetry. Only the naturally occurring D(+)- stereoisomer (with R configuration) is biologically active and the L(-)-stereoisomer (with S configuration) is inactive. When its carboxylate functional group is attached through an amide linkage with β-mercaptoethylamine, it is known as pantetheine (also spelled pantotheine). The biologically active form of pantothenic acid, CoA, is formed when the terminal alcoholic function of pantetheine is attached to ADP 3'-phosphate. | | Biochem/physiol Actions | The calcium salts of panthenol are commonly used for pharmaceutical preparations. Pantothenate is a component of coenzyme A and is useful in its synthesis. Pantothenic acid is also involved in the synthesis of heme, cholesterol and fatty acids. Since vitamin B5 is found in all foods, its deficiency is not commonly observed. | | 臨床応用 | The only therapeutic indication for pantothenic acid is intreatment of a known or suspected deficiency of this vitamin.Because of the ubiquitous nature of pantothenic acid, deficiencystates of this vitamin are only seen experimentally byuse of synthetic diets devoid of the vitamin, by use of thevitamin antagonist, ω-methylpantothenic, or both. In a 1991review, Tahiliani and Beinlich described that the mostcommon symptoms associated with pantothenic acid deficiencywere headache, fatigue, and a sensation of weakness.Sleep disturbances and gastrointestinal disturbances, amongothers, were also noted. The most likely setting for pantothenicacid deficiency is in the setting of alcoholism wherea multiple vitamin deficiency exists confounding the exactrole of the pantothenic acid deficiency as compared to theother vitamins. Because a deficiency of a single B vitamin israre, pantothenic acid is commonly formulated in multivitaminor B-complex preparations. | | 安全性プロファイル | Moderately toxic by
intraperitoneal, subcutaneous, and
intravenous routes. Mildly toxic by
ingestion. A vitamin. See also CALCIUM
COMPOUNDS. When heated to
decomposition it emits toxic fumes of NOx. | | 純化方法 | The salt crystallises as needles from MeOH, EtOH or isoPrOH (with 0.5mol of isoPrOH). It is moderately hygroscopic. The S-benzylisothiuronium salt has m 151-152o (149o when crystallised from Me2CO). [Kagan et al. J Am Chem Soc 79 3545 1957, Wilson et al. J Am Chem Soc 76 5177 1954, Stiller & Wiley J Am Chem Soc 63 1239 1941, Beilstein 4 IV 2569.] |
|