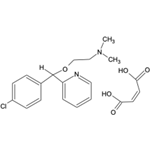
- Carbinoxamine maleate
-
- $0.00 / 1KG Ton
-
2023-09-08
- CAS:3505-38-2
- Min. Order: 1KG Ton
- Purity: USP43 98%+
- Supply Ability: 2 tons/month
|
| | CARBINOXAMINE MALEATE SALT Basic information |
| | CARBINOXAMINE MALEATE SALT Chemical Properties |
| Hazard Codes | T | | Risk Statements | 25-36/37/38 | | Safety Statements | 26-36/37/39-45 | | RIDADR | 3249 | | WGK Germany | 3 | | RTECS | US6350000 | | HazardClass | 6.1(b) | | PackingGroup | III | | HS Code | 2933399090 | | Toxicity | LD50 in mice (mg/kg): 166 i.p. (Cahen) |
| | CARBINOXAMINE MALEATE SALT Usage And Synthesis |
| Description | Carbinoxamine is a competitive histamine H1 receptor antagonist (Ki = 2.3 nM) and first generation antihistamine. It also competes with [3H]diltiazem, an L-type calcium channel blocker, for binding to the benzothiazepine site on rat cardiomyocytes (Ki = 1.08 nM). Carbinoxamine decreases negative inotropic activity in isolated guinea pig left atria by 76% (EC50 = 250 nM) and negative chronotropic activity in guinea pig spontaneously beating isolated right atria (EC50s = 250 and 480 nM, respectively) by 48% compared with control. Formulations containing carbinoxamine have been used in the treatment of allergic rhinitis. | | Chemical Properties | Off-White Solid | | Originator | Clistin,McNeil,US,1953 | | Uses | antihistaminic | | Uses | Analgesic and anti-inflammatory compound | | Definition | ChEBI: The maleic acid salt of carbinoxamine. An ethanolamine-type antihistamine, used for treating hay fever, as well as mild cases of Parkinson's disease. | | Manufacturing Process | As described in US Patent 2,800,485 a solution of p-chlorophenylmagnesium
bromide is prepared by adding dropwise a solution of 230 g (1.2 mols) of p-bromochlorobenzene in 900 cc of anhydrous ether to 26.7 g (1.1 g-atoms) of magnesium suspended in 100 cc of anhydrous ether containing a small crystal
of iodine. To this solution, 107 g (1 mol) of 2-pyridinealdehyde are added
slowly with stripping at a rate to maintain refluxing. The reaction mixture is
then stirred for one hour at room temperature. The mixture is then poured
onto an equal volume of crushed ice and water and acidified with concentrated
hydrochloric acid. The ether layer is removed. The aqueous layer is made
basic with ammonia and extracted with ether. The ether solution is evaporated
and the residue dried by addition of benzene and removal by distillation to
give 208 g (95%) of solid alpha-(p-chlorophenyl)-2-pyridinemethanol melting
at 78° to 80°C. The p-chlorophenyl pyridinemethanol may alternatively be
prepared from 4-chloroacetophenone, pyridine and granular aluminum as
described in US Patent 2,606,195. In either case, the synthesis then proceeds
as described in US Patent 2,800,485.
A solution of 219 g (1 mol) of α-(p-chlorophenyl)-2-pyridinemethanol in one
liter of dry toluene is heated to 100°C with stirring. Twenty-three grams (1 g-atom) of sodium are then added in portions. After all the sodium has reacted,
a dried solution of 2-dimethylaminoethyl chloride in benzene is added. This
benzene solution is prepared by dissolving 173 g (1.2 mols) of 2-
dimethylaminoethyl chloride hydrochloride in the minimum amount of water,
adding 500 cc of benzene followed by 300 g of sodium carbonate decahydrate,
stirring, separating the benzene layer and drying.
The mixture is refluxed with stirring for ten hours, cooled and filtered. The
filtrate is extracted three times with 200 cc portions of 6 N acetic acid. The
aqueous acetic acid solution is then made strongly basic with 10% sodium
hydroxide solution, and extracted three times with 200 cc portions of ether.
The ether extract is dried with anhydrous sodium sulfate, stirred with 5 g of
activated carbon and filtered to provide 2-[p-chloro-α(2-dimethylaminoethoxy)
benzyl]pyridine in solution. Addition of a solution of 116 g (1 mol) of maleic
acid in 1,500 cc of ether gives 323 g (79%) of solid which, on recrystallization
from ethyl acetate, gives white solid 2-[p-chloro-α(2-dimethylaminoethoxy)
benzyl]pyridine maleate melting at 117° to 119°C. | | Brand name | Clistin (Ortho-
McNeil). | | Therapeutic Function | Antihistaminic | | General Description | Carbinoxamine is availableas a bitter bimaleate salt, (d, l)-2-[p-chloro- -[2-(dimethylamino)ethoxy]benzyl]pyridine bimaleate (Clistin), which isa white crystalline powder that is very soluble in water andfreely soluble in alcohol and in chloroform. The pH of a 1%solution is between 4.6 and 5.1.
Carbinoxamine is a potent antihistaminic and is availableas the racemic mixture. It differs structurally from chlorpheniramineonly in having an oxygen atom separate theasymmetric carbon atom from the aminoethyl side chain. Themore active levo isomer of carbinoxamine has the (S) absoluteconfiguration and can be superimposed on the moreactive dextro isomer (S configuration) of chlorpheniramine. |
| | CARBINOXAMINE MALEATE SALT Preparation Products And Raw materials |
|