- Ethyl Mesilate
-

- $11.00 / 1kg
-
2023-02-13
- CAS:62-50-0
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 50MT
|
| | Ethyl methanesulfonate Basic information |
| Product Name: | Ethyl methanesulfonate | | Synonyms: | ENT 26396;ent26396;Ethyl methansulphonate;ethylmethansulfonate;ethylmethansulphonate;Ethylmethylsulfonate;Half-Myleran;Methanesulphonic acid ethyl ester | | CAS: | 62-50-0 | | MF: | C3H8O3S | | MW: | 124.16 | | EINECS: | 200-536-7 | | Product Categories: | | | Mol File: | 62-50-0.mol | 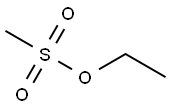 |
| | Ethyl methanesulfonate Chemical Properties |
| Melting point | <25 °C | | Boiling point | 85-86 °C/10 mmHg (lit.) | | density | 1.206 g/mL at 20 °C | | vapor pressure | 0.27 hPa (25 °C) | | refractive index | n20/D 1.418(lit.) | | Fp | 100 °C | | storage temp. | 2-8°C | | solubility | 50-100g/l | | form | liquid | | color | Colourless to Pale Yellow | | Water Solubility | Miscible with water. | | Sensitive | Moisture Sensitive | | Merck | 14,3827 | | BRN | 773969 | | Stability: | Stable. Combustible. Incompatible with water, alkalies, oxidizing agents. | | InChIKey | PLUBXMRUUVWRLT-UHFFFAOYSA-N | | CAS DataBase Reference | 62-50-0(CAS DataBase Reference) | | IARC | 2B (Vol. 7, Sup 7) 1987 | | NIST Chemistry Reference | Methanesulfonic acid, ethyl ester(62-50-0) | | EPA Substance Registry System | Ethyl methanesulfonate (62-50-0) |
| | Ethyl methanesulfonate Usage And Synthesis |
| Chemical Properties | Ethyl methane sulfonate is a clear liquid. | | Chemical Properties | colourless liquid | | Uses | Experimentally as mutagen, teratogen and brain carcinogen. | | Uses | Ethyl methanesulfonate is used as mutagen for both mammalian and plant cells. It finds application as a model alkylating agent in the study of deoxyribonucleic acid (DNA) repair processes. | | Definition | ChEBI: A methanesulfonate ester resulting from the formal condensation of methanesulfonic acid with ethanol. | | General Description | Clear colorless liquid. Denser than water. | | Air & Water Reactions | Water soluble. | | Reactivity Profile | Ethyl methanesulfonate alkylates nucleophiles such as hydroxy, amino and sulfhydryl groups in model and biological materials. Is hydrolyzed by excess aqueous alkali to non-corrosive and non-toxic products. Is hydrolyzed by water to a highly corrosive product . | | Fire Hazard | Ethyl methanesulfonate is combustible. | | Biochem/physiol Actions | Ethyl methanesulfonate is a DNA ethylating agent, mutagenic to plants and animals and carcinogenic in mammals. It has been used as a model alkylating agent in studies of DNA repair processes. EMS induces base substitutions of guanine-cytosine (G/C) to adenine-thymine (A/T). EMS also generates point mutations and single nucleotide polymorphisms in genomes. EMS is potential chemical mutagen used for inducing mutation in rice, wheat and Arabidopsis thaliana. | | Safety Profile | Confirmed carcinogen
with experimental carcinogenic,
neoplas tigenic, tumorigenic, and teratogenic
data. Poison by ingestion and intraperitoneal
routes. Experimental reproductive effects.
Human mutation data reported. When
heated to decomposition it emits toxic
fumes of SOx. See also SULFONATES and
ESTERS. | | Potential Exposure | Used as a research tool for mutagenesis and carcinogenesis studies. Was considered as a possible
human male contraceptive. Also considered as a reversible
male hemosterilant for insects and mammalian pests. | | Carcinogenicity | Ethyl methanesulfonate is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals. | | Shipping | UN2810 Toxic liquids, organic, n.o.s., Hazard
Class: 6.1; Labels: 6.1-Poisonous materials, Technical
Name Required. Military driver shall be given full and
complete information regarding shipment and conditions in
case of emergency. AR 50-6 deals specifically with the
shipment of chemical agents. Shipments of agent will be
escorted in accordance with AR 740-32. | | Incompatibilities | Vapors may form explosive mixture with
air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep
away from alkaline materials, strong bases, strong acids,
oxoacids, and epoxides. Contact with moisture may cause
hydrolysis or other forms of decomposition |
| | Ethyl methanesulfonate Preparation Products And Raw materials |
|