- 5-HYDROXY-L-TRYPTOPHAN
-

- $1.00 / 1g
-
2024-09-23
- CAS:314062-44-7
- Min. Order: 1g
- Purity: 99.9+%
- Supply Ability: 20T
- L-5HTP
-

- $100.00 / 1kg
-
2024-09-19
- CAS:314062-44-7
- Min. Order: 1kg
- Purity: >99%
- Supply Ability: 20tons
- 5-HTP
-

- $0.00 / 1KG
-
2024-09-18
- CAS:314062-44-7
- Min. Order: 1KG
- Purity: 98.0%
- Supply Ability: 500kg/month
|
| | 5-hydroxy-l-tryptophan Basic information |
| Product Name: | 5-hydroxy-l-tryptophan | | Synonyms: | (S)-2-AMino-3-(5-hydroxy-1H-indol-3-yl)propanoic acid hydrate;5-HYDROXY-TRP-OH;5-HYDROXY-L-TRP-OH;5-HTP;(S)-2-AMINO-3-(5-HYDROXY-1H-INDOL-3-YL)-PROPIONIC ACID;C5-HYDROXY-L-TRYPTOPHAN;H-TRP(5-OH)-OH;H-5-HYDROXY-TRP-OH | | CAS: | 314062-44-7 | | MF: | C11H12N2O3 | | MW: | 220.22 | | EINECS: | 224-411-1 | | Product Categories: | | | Mol File: | 314062-44-7.mol | 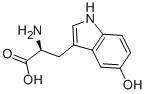 |
| | 5-hydroxy-l-tryptophan Chemical Properties |
| Melting point | 270 °C (dec.)(lit.) | | density | 0.902 g/mL at 25 °C(lit.) | | refractive index | n20/D 1.4850(lit.) | | Fp | 175 °F | | storage temp. | Keep in dark place,Inert atmosphere,2-8°C | | solubility | H2O: 10 mg/mL | | form | solid | | color | white | | InChI | InChI=1/C11H12N2O3/c12-9(11(15)16)3-6-5-13-10-2-1-7(14)4-8(6)10/h1-2,4-5,9,13-14H,3,12H2,(H,15,16)/t9-/s3 | | InChIKey | LDCYZAJDBXYCGN-DJEYLCQNNA-N | | SMILES | C12C=C(C=CC=1NC=C2C[C@H](N)C(=O)O)O |&1:10,r| | | CAS DataBase Reference | 314062-44-7(CAS DataBase Reference) |
| Hazard Codes | Xn | | Risk Statements | 22 | | Safety Statements | 36 | | RIDADR | UN 2811 6.1/PG 3 | | WGK Germany | 3 | | RTECS | YN7110000 | | F | 3-10 |
| | 5-hydroxy-l-tryptophan Usage And Synthesis |
| Description | 5-hydroxy-l-tryptophan (5-HTP) is a chemical byproduct of the protein building block L-tryptophan. It is produced commercially from the seeds of an African plant known as Griffonia simplicifolia. In vivo, it is produced from tryptophan by tryptophan hydroxylase (TPH), and its decarboxylation yields serotonin (5-hydroxytryptamine, 5-HT), a monoamine neurotransmitter involved in the modulation of mood, cognition, reward, learning, memory, sleep, and numerous other physiological processes. 5-HTP is both a drug and a natural component of some dietary supplements, and occurrence in both synthetic tryptophan and 5-HTP of toxic impurities has caused eosinophilia myalgia syndrome cases[1]. | | History |
5-hydroxy-l-tryptophan (5-HTP) has been used clinically for more than 30 yr. Sales and awareness of 5-HTP increased in 1989 after the Food and Drug Administration (FDA) banned its precursor, L-tryptophan, because of its association with eosinophilia-myalgia syndrome. Since then, 5-HTP has been marketed as a safe alternative to L-tryptophan.
| | Uses | 5-hydroxy-l-tryptophan is metabolized into serotonin and is thought to alleviate depression by enhancing serotonin neurotransmission. It is also used to treat fibromyalgia, insomnia, binge-eating, attention deficit disorder, and chronic headaches. | | Side effects | Administration of 5-hydroxy-l-tryptophan in patients with major depression who received selective serotonin reuptake inhibitors like fluoxetine resulted in significantly increased levels of cortisol and prolactin in plasma compared with untreated depressed patients, suggesting that the adverse effect of 5-hydroxy-l-tryptophan was potentiated by fluoxetine. The main side effects of 5-hydroxy-l-tryptophan are dose-related, and sleepiness, fatigue, nausea, or vomiting are the most cited. | | Metabolism |
5-Hydroxy-l-tryptophan is a precursor for melatonin (N-acetyl-5-methoxytryptamine, a regulatory hormone of circadian rhythm) that is synthesized from serotonin via N-acetyl-serotonin. It is transported to various body tissues, including the brain, where it is transformed into serotonin. The absorption of 5-hydroxy-tryptophan and its decarboxylation to serotonin are processes of variable efficiencies (47%–84%). The serotonin is metabolized sequentially to its inactive metabolites, and it is excreted in the form of 5-hydroxyindoleacetic acid.
| | References | [1] Maffei, Massimo E. "5-Hydroxytryptophan (5-HTP): Natural Occurrence, Analysis, Biosynthesis, Biotechnology, Physiology and Toxicology." International Journal of Molecular Sciences 22.1(2021):181. |
| | 5-hydroxy-l-tryptophan Preparation Products And Raw materials |
|