almecillin manufacturers
- almecillin USP/EP/BP
-
- $1.10 / 1g
-
2021-06-28
- CAS:87-09-2
- Min. Order: 1g
- Purity: 99.9%
- Supply Ability: 100 Tons Min
|
| | almecillin Basic information |
| Product Name: | almecillin | | Synonyms: | almecillin;PenicillinO;(6R)-6-[N-[2-(Allylthio)acetyl]amino]penicillanic acid;6α-[[(2-Propenylthio)acetyl]amino]penicillanic acid;Allylthiomethylpenicillin;Penicillin AT;(2S,5R,6R)-3,3-dimethyl-7-oxo-6-(2-prop-2-enylsulfanylethanoylamino)-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid;(2S,5R,6R)-3,3-dimethyl-7-oxo-6-[(2-prop-2-enylsulfanylacetyl)amino]-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid | | CAS: | 87-09-2 | | MF: | C13H18N2O4S2 | | MW: | 330.42 | | EINECS: | | | Product Categories: | | | Mol File: | 87-09-2.mol | 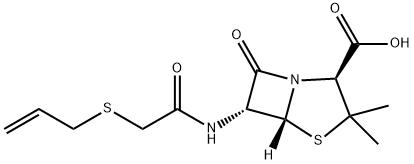 |
| | almecillin Chemical Properties |
| Boiling point | 227.2°C (rough estimate) | | density | 1.3468 (rough estimate) | | refractive index | 1.6510 (estimate) |
| | almecillin Usage And Synthesis |
| Originator | Cero-O-Cillin,Upjohn,US,1950 | | Definition | ChEBI: A penicillin where the side-chain N-acyl group is specified as allylmercaptoacetyl. Antibiotic isolated from Penicillium chrysogenum. | | Manufacturing Process | A culture medium is prepared in the following proportions:
Lactose 125 g Corn steep solids 150 g Calcium carbonate 25 g N-(2-Hydroxyethyl)-allylmercaptoacetamide 0.140 g Water 5,000 cc
The culture medium is distributed in 200 cc portions in 1 liter Erlenmeyer flasks, sterilized, inoculated with a spore suspension of Penicillium mold strain Q-176, and stoppered with cotton plugs. The flasks are maintained at a temperature of about 23°C to 26°C and shaken constantly for five days. The flask contents are then filtered to remove the mold mycelium, the filtrate cooled to about 0°C, acidified to about pH 2.2 with o-phosphoric acid and shaken with an equal volume of amyl acetate. The amy acetate layer is separated and extracted with three 100 cc portions of cold water to which cold N/10 sodium bicarbonate solution is added during the course of each extraction until a pH of about 7.1 to 7.3 is attained in the aqueous phase. The aqueous extracts are combined, cooled to about 0°C. acidified to about pH 2.2 with o-phosphoric acid and extracted with three 100 cc portions of ether. The ether extracts are combined, and are passed through a chromatographic type silica adsorption column about 30 mm in diameter and 300 mm long, and containing a pH 6.2 phosphate buffer. The silica column is developed by percolation with six 100 cc portions of ether containing successively increasing amounts of methanol in the order of 0.5, 1.5%, 2, 2.5, and 3 percent.
The developed silica column is divided into about 12 equal sections and each section is eluted with three 30 cc portions of M/15 phosphate buffer of pH 7.0. The eluates are assayed bacteriologically to determine their penicillin content. Most of the antibiotic activity originates in a single bank in the silica column and results from the presence of allylmercaptomethylpenicillin. The eluates obtained from this band are combined, cooled to about 0°C, acidified to about pH 2.2and extracted with three 50 cc portions of chloroform. The combined chloroform extracts are then passed through a silica adsorption column containing a pH 6.2 phosphate buffer. This silica gel column is developed by percolation with three 100 cc portions of chloroform containing successively increasing amounts of methanol in the order of 1, 2 and 3 percent. The developed silica column is then divided into 12 equal sections and each section is eluted with three 30 cc portions of M/15 phosphate buffer of pH 7.0.Again, most of the total antibiotic activity originates in a single band in the silica column. The eluates obtained by extraction of the silica column sections which comprise this band are combined, cooled to about 0°C, acidified to about pH 2.2 and extracted with three 100 cc portions of ether. The ether extracts are combined and extracted with about 75 cc of a cool dilute aqueous solution of sodium hydroxide to which N/10 sodium hydroxide solution is added during the course of the extraction so that a final pH of about 7.0 is obtained in the aqueous phase. From this aqueous solution the sodium salt of allylmercaptomethylpenicillin is separated, for example, by freezing and evaporation in vacuo from the frozen state. | | Therapeutic Function | Antibacterial |
| | almecillin Preparation Products And Raw materials |
|