| Company Name: |
TargetMol Chemicals Inc.
|
| Tel: |
4008200310 |
| Email: |
marketing@tsbiochem.com |
| Products Intro: |
Product Name:Abanoquil;Abanoquil
CAS:90402-40-7
Purity:98% Package:5 mg
|
|
| | 2-(6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl)-6,7-dimethoxy-quinolin-4-amine Basic information |
| Product Name: | 2-(6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl)-6,7-dimethoxy-quinolin-4-amine | | Synonyms: | 2-(6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl)-6,7-dimethoxy-quinolin-4-amine;2-(6,7-Dimethoxy-1,2,3,4-tetrahydroisoquinoline-2-yl)-6,7-dimethoxyquinoline-4-amine;4-Amino-6,7-dimethoxy-2-[(1,2,3,4-tetrahydro-6,7-dimethoxyisoquinolin)-2-yl]quinoline;4-Quinolinamine, 2-(3,4-dihydro-6,7-dimethoxy-2(1H)-isoquinolinyl)-6,7-dimethoxy- | | CAS: | 90402-40-7 | | MF: | C22H25N3O4 | | MW: | 395.45 | | EINECS: | | | Product Categories: | | | Mol File: | 90402-40-7.mol | 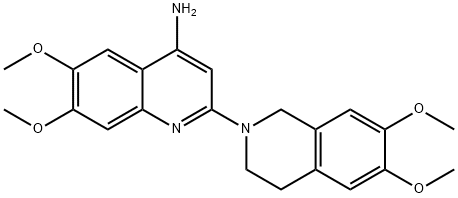 |
| | 2-(6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl)-6,7-dimethoxy-quinolin-4-amine Chemical Properties |
| Melting point | 229-230 °C | | Boiling point | 630.3±55.0 °C(Predicted) | | density | 1.246±0.06 g/cm3(Predicted) | | storage temp. | Store at -20°C | | pka | 9.30±0.61(Predicted) |
| | 2-(6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl)-6,7-dimethoxy-quinolin-4-amine Usage And Synthesis |
| Originator | Abanquil,Onbio Inc. | | Manufacturing Process | Synthesis of 1-(6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl)ethanone:
To a stirred solution of 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline (3.00 g,
15.4 mmol, 1.00 equiv.) in anhydrous pyridine (100 mL) under argon at room
temperature was added acetic anhydride (14.5 mL, 154 mmol, 10.0 equiv.)
over 15 min. The resulting mixture was stirred at room temperature for 2 h,
and then at reflux for 6 h. The volatiles were removed by rotary evaporation
at 80°C under high vacuum. The residue was flash chromatographed on silica
gel (MeOH-CH2Cl2 8:92) to afford 3.21 g (89%) of 1-(6,7-dimethoxy-3,4-
dihydro-1H-isoquinolin-2-yl)ethanone as a viscous brown oil. The H-NMR
spectrum reflected the presence of two slowly interconverting conformers in a
ratio of 1.2:1 at room temperature.
Synthesis of 2-[1-(6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-
yl)ethylidineamino]-4,5-dimethoxybenzonitrile:
To a stirred solution of 1-(6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-
yl)ethanone (1.00 g, 4.25 mmol, 1.00 equiv.) in CHCl3 at room temperature
under argon was added POCl3 (143 μL, 1.53 mmol, 0.36 equiv.). After 10 min,
2-amino-4,5-dimethoxybenzonitrile (763 mg, 4.28 mmol, 1.01 equiv.) was
added and the mixture was heated at reflux overnight. The mixture was
cooled to room temperature and poured into 1 M aq. NaOH solution (50 mL),
and the aqueous phase was extracted with CH2Cl2. The combined organic
solutions were dried over MgSO4 and concentrated. The residue was flash
chromatographed on silica gel (MeOH-CH2Cl2, 2 5:95) to afford 482 mg (28%)
of 2-[1-(6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl)ethylidineamino]-4,5-
dimethoxybenzonitrile as an yellow solid.
Synthesis of 2-(6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl)-6,7-
dimethoxyquinolin-4-ylamine hemifumarate hydrate (abanoquil):
To a stirred solution of 2-[1-(6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-
yl)ethylidineamino]-4,5-dimethoxybenzonitrile (471 mg, 1.19 mmol, 1.00
equiv.) in refluxing anhydrous N,N-dimethylacetamide (24 mL) under argon
was added ZnCl2 (339 mg, 2.49 mmol, 2.10 equiv.) in three portions over 1
h. The solvent was removed by distillation at 70°C under high vacuum. Ether
(40 mL) was added to the residue, which was broken up with a stirring rod,
and the mixture was stirred at 0°C to precipitate the product. The supernatant
was discarded, and the precipitate was washed twice more at 0°C with ether.
The solid residue was stirred with 1 M aq. NaOH (25 mL) and CH2Cl2 (25 mL)
for 10 min, and the aqueous phase was extracted with CH2Cl2. The combined
organic solutions were dried over MgSO4 and concentrated to give 493 mg of
brown oil, which was flash chromatographed on silica gel (MeOH-CH2Cl2,12:88
followed by 2-propylamine-CH2Cl2, 5:95) to afford 151 mg (38%) of 2-(6,7-
dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl)-6,7-dimethoxyquinolin-4-ylamine
as a tan solid.
To a solution of 2-(6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl)-6,7-
dimethoxyquinolin-4-ylamine (150 mg) in hot CH2Cl2 (4.5 mL) and MeOH (1.5
mL) was added a solution of fumaric acid (22.8 mg, 0.196 mmol, 0.50 equiv.)
in hot MeOH (3.0 mL). The resulting mixture was concentrated and the
product was recrystallized from MeOH with hot filtration to afford, after
filtration, 85 mg of light brown solid: m.p. 239-240°C. | | Therapeutic Function | Antiarrhythmic, Coronary vasodilator |
| | 2-(6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl)-6,7-dimethoxy-quinolin-4-amine Preparation Products And Raw materials |
| Raw materials | Acetic anhydride-->-fluoro benzophenone-->Sodium hydroxide-->7-METHOXY-1,2,3,4-TETRAHYDRO-ISOQUINOLINE HYDROCHLORIDE-->6,7-DIMETHOXY-1,2,3,4-TETRAHYDROISOQUINOLINE-->2-Amino-4,5-dimethoxybenzonitrile-->6,7-Dimethoxy-1,2,3,4-tetrahydroisoquinoline hydrochloride-->Phosphorus oxychloride-->Zinc chloride-->Fumaric acid |
|