- Abiraterone acetate
-
- $1.00 / 1kg
-
2024-04-26
- CAS:154229-18-2
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 200
- Abiraterone Acetate
-

- $30.00 / 1kg
-
2024-04-26
- CAS:154229-18-2
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: 2000kg
- Stanozolol
-

- $30.00 / 1kg
-
2024-04-26
- CAS:154229-18-2
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: 2000kg
|
| | Abiraterone acetate Basic information |
| Product Name: | Abiraterone acetate | | Synonyms: | Abiraterone acetate 99.5%;Abiraterone acetate(CB7630);3β-acetoxy-17-(3-pyridyl)androsta-5,16-diene;2-((3S,8R,9S,10R,13S,14S)-10,13-diMethyl-17-(pyridin-3-yl)-2,3,4,7,8,9,10,11,12,13,14,15-dodecahydro-1H-cyclopenta[a]phenanthren-3-yl)acetate;Abiraterone acetate ester;Androsta-5,16-dien-3-ol,17-(3-pyridinyl)-, acetate (ester), (3b)-;Abiraterone Acetate impurity;(3β)-17-(3-pyridinyl) androsta-5,16-dien-3-yl acetate | | CAS: | 154229-18-2 | | MF: | C26H33NO2 | | MW: | 391.55 | | EINECS: | 620-314-7 | | Product Categories: | Inhibitors;Inhibitor;Final material;Anti-cancer & immunity;DQM;API;Intermediates & Fine Chemicals;Pharmaceuticals;Steroids;154229-18-2 | | Mol File: | 154229-18-2.mol | 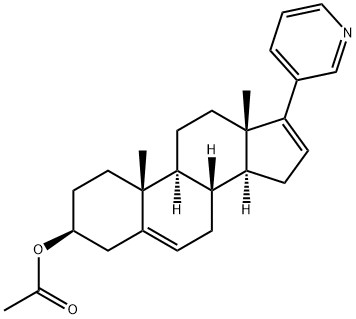 |
| | Abiraterone acetate Chemical Properties |
| Melting point | 127-130°C | | Boiling point | 506.7±50.0 °C(Predicted) | | density | 1.14±0.1 g/cm3(Predicted) | | storage temp. | -20°C | | solubility | Chloroform (Slightly), DMSO (Sparingly), Methanol (Sparingly) | | pka | 5.31±0.12(Predicted) | | form | powder | | color | white to beige |
| WGK Germany | 3 | | RTECS | BV7992100 | | HS Code | 2937290000 |
| | Abiraterone acetate Usage And Synthesis |
| Description | In April 2011, the United States FDA approved abiraterone acetate
(CB7630) in combination with the steroid prednisone for the treatment
of metastatic castration-resistant prostate cancer (mCRPC) for patients
who were previously treated with a docetaxel containing regimen for
late-stage disease. Abiraterone acetate affects prostate, testicular, and adrenal androgens by irreversibly inhibiting
both the lyase and hydroxylase activity of cytochrome P450 17A
(CYP17) signaling pathways (IC50's of 2.9 and 4 nM, respectively) thereby
decreasing testosterone levels.Most common serious adverse events for abiraterone acetate versus placebo included fluid retention (30.5% vs. 22.3%), hypokalemia (17.1% vs. 8.4%), hypertension (9.7% vs. 7.9%), hepatic transaminase abnormalities (10.4% vs. 8.1%), and cardiac abnormalities (13.3% vs. 10.4%). | | Chemical Properties | Off-White Solid | | Originator | Institute of Cancer Research, London (United Kingdom) | | Uses | A novel steroidal inhibitor of human Cytochrome P450(17a-Hydroxylase-C17,20-lyase): potential agent for the treatment of prostatic cancer. | | Uses | Abiraterone acetate was approved by the U.S. Food and Drug
Administration (FDA) in April 2011 for the treatment of castrationresistant
prostate cancer. The drug, marketed under the trade name
Zytiga, was originally discovered by researchers at the Cancer Research
UK Centre for Cancer Therapeutics in 1990, developed by Cougar
Biotechnology, and ultimately commercialized by Johnson &
Johnson after its acquisition of Cougar in 2009. Abiraterone acetate
inhibits CYP17A1—an enzyme expressed in testicular, adrenal, and
prostatic tumor tissues—which has been implied in the production of
testosterone and the proliferation of such tumor cell lines. | | Uses | Abiraterone acetate is a novel steroidal inhibitor of human Cytochrome P450 (17α-Hydroxylase-C17,20-lyase): potential agent for the treatment of prostatic cancer. | | Definition | ChEBI: A sterol ester obtained by formal condensation of the 3-hydroxy group of abiraterone with the carboxy group of acetic acid. A prodrug that is converted in vivo to abiraterone. Used for treatment of metastatic castrate-resistant prostate cance
. | | Brand name | Zytiga | | Biochem/physiol Actions | Abiraterone acetate is a prodrug of abiraterone, which is a potent, selective, and orally bioavailable inhibitor of CYP17A1 (CYP450c17), an enzyme that catalyzes two key serial reactions (17α hydroxylase and 17,20 lyase) in androgen and estrogen biosynthesis resulting in the formation of DHEA and androstenedione, which may ultimately be metabolized into testosterone. CYP17 is the key enzyme for androgen biosynthesis in both the testes and adrenals, so its inhibition should stop the production of androgens in both places. Abiraterone acetate is used for the treatment of metastatic castration-resistant prostate cancer. Abiraterone acetate possesses significant antitumor activity in post-docetaxel patients with CRPC (castration-resistant prostate cancer). It is highly essential for androgen biosynthesis in the testes, adrenal glands, and prostate tissue. | | Clinical Use | Hormone antagonist:
Treatment of metastatic prostate cancer | | Synthesis | The most convenient synthesis for scale-up will be highlighted
from two published syntheses. Commercially available
androstenolone 1 was acylated with acetic anhydride in the
presence of boron trifluoride-diethyl etherate to give a near quantitative
yield of acetate 2. The conversion of ketone 2 to vinyl triflate
3 involved careful selection of base to prevent elimination of the acetate
group. To this extent, subjection of 2 to triflic anhydride in
dichloromethane at ambient temperature followed by slow addition
of triethylamine minimized undesired side products and delivered
triflate 3 in 60% isolated yield. Subsequent Suzuki coupling with
diethylborane 4 under standard conditions ultimately furnished
abiraterone acetate (I) in 75% yield. 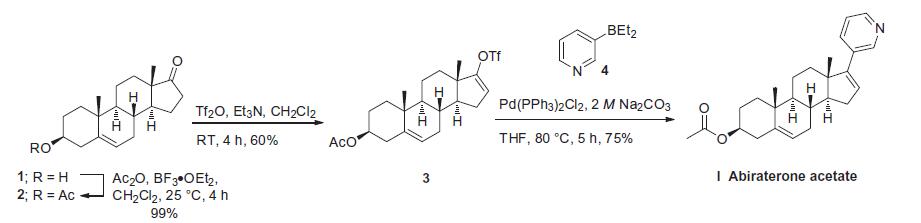
| | target | P450 (e.g. CYP17) | | Drug interactions | Potentially hazardous interactions with other drugs
Antibacterials: concentration possibly reduced by
rifabutin and rifampicin - avoid.
Antidepressants: concentration possibly reduced by
St John’s wort - avoid.
Antiepileptics: concentration possibly reduced
by carbamazepine, fosphenytoin, phenobarbital,
phenytoin and primidone - avoid. | | Metabolism | Abiraterone acetate is hydrolysed to abiraterone, which
then undergoes metabolism including sulphation,
hydroxylation and oxidation mainly in the liver to form
inactive metabolites. About 88% of a dose is excreted in
the faeces, of which about 55% is unchanged abiraterone
acetate and about 22% is abiraterone; about 5% of a dose
is excreted in the urine. | | storage | Store at -20°C |
| | Abiraterone acetate Preparation Products And Raw materials |
|