|
ChemicalBook Optimization Suppliers |
|
| 融点 | 165-169 °C (lit.) | | 沸点 | 306.47°C (rough estimate) | | 比重(密度) | 1.1999 (rough estimate) | | 屈折率 | 1.5460 (estimate) | | 闪点 | 171°C | | 貯蔵温度 | -20°C | | 溶解性 | DMSO:30.0(Max Conc. mg/mL);171.25(Max Conc. mM) | | 外見 | crystalline | | 酸解離定数(Pka) | 4.75(at 25℃) | | 色 | off-white to tan | | 水溶解度 | Soluble in ethanol (50 mg/ml), methanol, DMSO, and chloroform (sparingly). Insoluble in water. | | Decomposition | 167 ºC | | Sensitive | Light Sensitive | | Merck | 14,4964 | | BRN | 143358 | | 安定性: | Stable. Incompatible with strong oxidizing agents. Light sensitive. | | LogP | 1.410 | | CAS データベース | 87-51-4(CAS DataBase Reference) | | NISTの化学物質情報 | Indole-3-acetic acid(87-51-4) | | EPAの化学物質情報 | Indole-3-acetic acid (87-51-4) |
| 主な危険性 | Xi | | Rフレーズ | 36/37/38 | | Sフレーズ | 22-24/25 | | WGK Germany | 3 | | RTECS 番号 | NL3150000 | | F | 8-10-23 | | Hazard Note | Irritant | | TSCA | Yes | | HSコード | 29339990 | | 毒性 | LD50 intraperitoneal in mouse: 150mg/kg |
| | 3-インドール酢酸 Usage And Synthesis |
| 外観 | 白色~赤褐色, 結晶~粉末 | | 定義 | 本品は、次の化学式で表される複素環式化合物である。 | | 溶解性 | アルカリ水溶液, エタノール, アセトンに易溶。エーテルに易溶、水, ベンゼンに難溶。 | | 化粧品の成分用途 | 皮膚コンディショニング剤 | | 化学的特性 | white to tan crystals | | 使用 | It is applied as plant growth hormone. is an inducer of plant cell elongation and division shown to cause uncontrolled growth. | | 使用 | Plant growth regulator. | | 定義 | ChEBI: A monocarboxylic acid that is acetic acid in which one of the methyl hydrogens has been replaced by a 1H-indol-3-yl group. | | 生合成 | 3-Indolylacetic acid is biosynthesised in plants from tryptophan by two pathways, the indolylpyruvic acid pathway being quantitatively the more important. Experiments with tomato shoots have shown the existence of a tryptophan transaminase, which catalyses the formation of indolylpyruvic acid, and a tryptophan decarboxylase, which catalyses the formation of tryptamine. The decarboxylation of indolylpyruvic acid is catalysed by indolylpyruvate decarboxylase, while indolylacetaldehyde dehydrogenase catalyses the oxidation of indolylacetaldehyde to indolylacetic acid.
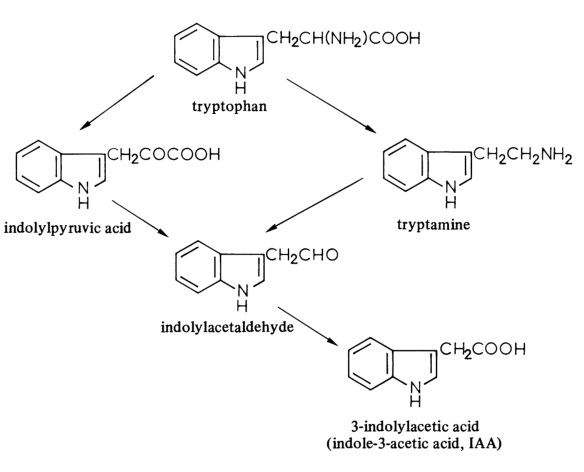
The biosynthesis of 3-indolylacetic acid | | 生物学の機能 | 3-Indolylacetic acid (indole-3-acetic acid, IAA) is one of the auxins, which together with the gibberellins and abscisic acid, cyto- kinins and ethylene are hormones regulating the growth and development of plants. IAA is a ubiquitous constituent of higher plants and the most important auxin. Some other, non-indolic compounds, including phenyl- acetic acid biosynthesised in plants from phenylalanine, have similar properties and synthetic auxins have also been prepared.
In the plant, IAA conjugates with many compounds, including glucose and other sugars, and with aspartic and glutamic acids. This is probably a way of storing the hormone for future use.
IAA initiates many growth effects in plants, including geotropism and phototropism, development of the ovary, division of cells, enlargement in callus tissue, root formation and apical dominance. When fed to plants, the hormone causes growth up to a maximum, which depends on the type of tissue being fed, and thereafter inhibits further growth, probably through the formation of ethylene, which is growth-inhibitory. Stern tissues tolerate the highest levels of IAA and root tissues the lowest. In the plant, the most active sites of IAA synthesis are the young, expanding leaves. | | 一般的な説明 | 3-Indoleacetic acid is a highly effective, growth promotor in lower plant life, formed by a variety of fungi, including yeast and has been isolated from Aspergillus niger and Rhizopus sp. It is commonly employed in horticulture and industry. | | 农业用途 | Indoleacetic acid (IAA), synthesized in the plant shoot tips, is a naturally occurring auxin. It is a plant growth promoter. | | 貯蔵 | Store at -20°C | | 純化方法 | Recrystallise heteroauxin from EtOH/water [James & Ware J Phys Chem 89 5450 1985]. [Beilstein 22 III/IV 65.] Alternatively recrystallise 30g of the acid with 10g of charcoal in 1L of hot water, filter and cool when 22g of colourless acid separate. Dry it and store it in a dark bottle away from direct sunlight [Johnson & Jacoby Org Synth Coll Vol V 654 1973]. The picrate has m 178-180o. [Beilstein 22 H 66, 22 I 508, 22 II 50, 22 III/IV 1088.] It is a plant growth substance. |
|