| Company Name: |
Mainchem Co., Ltd.
|
| Tel: |
+86-0592-6210733 |
| Email: |
sale@mainchem.com |
| Products Intro: |
Product Name:Nonyl trichlorosilane
CAS:5283-67-0
|
| Company Name: |
Shaanxi Dideu Newmaterial Co., Ltd.
|
| Tel: |
029-63373950 15353716720 |
| Email: |
1052@dideu.com |
| Products Intro: |
Product Name:trichloro(nonyl)silane
CAS:5283-67-0
Purity:98% Package:25kg
|
|
| | Nonyl trichlorosilane Basic information |
| Product Name: | Nonyl trichlorosilane | | Synonyms: | Nonyl trichlorosilane;trichlorononylsilane;nonyltrichloro-silan;trichlorononyl-silan;Silane, trichlorononyl- | | CAS: | 5283-67-0 | | MF: | C9H19Cl3Si | | MW: | 261.69 | | EINECS: | 226-113-7 | | Product Categories: | | | Mol File: | 5283-67-0.mol | 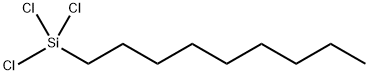 |
| | Nonyl trichlorosilane Chemical Properties |
| | Nonyl trichlorosilane Usage And Synthesis |
| Chemical Properties | Water-white liquid; pungent irritating
odor. Fumes readily in moist air. | | Chemical Properties | Nonyl trichlorosilane is a clear fuming liquid.
Irritating odor. | | General Description | Nonyl trichlorosilane is a colorless liquid with a pungent odor. Nonyl trichlorosilane is decomposed by water to hydrochloric acid with evolution of heat. Nonyl trichlorosilane is corrosive to metals and tissue. | | Reactivity Profile | Chlorosilanes, such as Nonyl trichlorosilane, are compounds in which silicon is bonded to from one to four chlorine atoms with other bonds to hydrogen and/or alkyl groups. Chlorosilanes react with water, moist air, or steam to produce heat and toxic, corrosive fumes of hydrogen chloride. They may also produce flammable gaseous H2. They can serve as chlorination agents. Chlorosilanes react vigorously with both organic and inorganic acids and with bases to generate toxic or flammable gases. | | Hazard | Strong irritant to skin and mucous membranes. | | Health Hazard | TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Contact with molten substance may cause severe burns to skin and eyes. Reaction with water or moist air will release toxic, corrosive or flammable gases. Reaction with water may generate much heat that will increase the concentration of fumes in the air. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution. | | Fire Hazard | Combustible material: may burn but does not ignite readily. Substance will react with water (some violently) releasing flammable, toxic or corrosive gases and runoff. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapors may travel to source of ignition and flash back. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water. | | Safety Profile | A corrosive irritant to
skin, eyes, and mucous membranes. When
heated to decomposition it emits toxic
fumes of Cl-. See also CHLOROSILANES. | | Potential Exposure | Used in silicone (polysiloxane)
manufacture. | | Shipping | UN1799 Nonyltrichlorosilane, Hazard Class: 8;
Labels: 8-Corrosive material. | | Incompatibilities | Incompatible with oxidizers (chlorates,
nitrates, peroxides, permanganates, perchlorates, chlorine,
bromine, fluorine, etc.); contact may cause fires or explosions.
Keep away from alkaline materials, strong bases,
strong acids, oxoacids, epoxides. Chlorosilanes react vigorously
with bases and both organic and inorganic acids generating
toxic and/or flammable gases. Chlorosilanes react
with water, moist air, or steam to produce heat and toxic,
corrosive fumes of hydrogen chloride. They may also produce
flammable gaseous hydrogen. Attacks metals in the
presence of moisture. |
| | Nonyl trichlorosilane Preparation Products And Raw materials |
|