- Atracurium Besylate
-

- $0.00 / 25Kg/Bag
-
2024-04-22
- CAS:64228-81-5
- Min. Order: 2Kg/Bag
- Purity: USP
- Supply Ability: 20 tons
- Atracurium besylate
-

- $40.00 / 1kg
-
2023-09-06
- CAS:64228-81-5
- Min. Order: 1kg
- Purity: 0.99
- Supply Ability: 10 tons
- Atracurium besylate
-

- $10.00 / 1kg
-
2023-07-31
- CAS:64228-81-5
- Min. Order: 10kg
- Purity: 99%
- Supply Ability: 20tons
|
| Product Name: | Atracurium besylate | | Synonyms: | BW-33A;2,2'-[1,5-pentanediylbis[oxy(3-oxo-3,1-propanediyl)]]bis[1-[(3,4-dimethoxyphenyl)methyl]-1,2,3,4-tetrahydro-6,7-dimethoxy-2-methyl-isoquinolinium dibenzenesulfonate;2,2’-[1,5-Pentanediylbis[oxy(3-oxcr3,1-propanediyl)]]bis[1-[(3,4-dimethoxyphenyl)methyl]-1,2,3,4-tetrahydro-6,7-dimethoxy-2-methylisoquinolinium]dibenzenesulfonate;51W-89:Nimbex;Atraeurium Besylate;Cisatracudum Besylate;BW-33A, Tracrium, 2,2μ-[1,5-Pentanediylbis[oxy(3-oxo-3,1-propanediyl)]]bis[1-[3,4-dimethoxyphenyl)methyl]-1,2,3,4-tetrahydro-6,7-dimethoxy-2-methylisoquinolinium]dibenzenesulfonate;Isoquinolinium, 2,2'-[1,5-pentanediylbis[oxy(3-oxo-3,1-propanediyl)]]bis[1-[(3,4-dimethoxyphenyl)methyl]-1,2,3,4-tetrahydro-6,7-dimethoxy-2-methyl-, dibenzenesulfonate | | CAS: | 64228-81-5 | | MF: | C65H82N2O18S2 | | MW: | 1243.48 | | EINECS: | 264-743-4 | | Product Categories: | Acetylcholine receptor;Pharmaceuticals;Inhibitors;Analgesic series;SYNTETRIN;API;Neuromuscular Blocking Agent;Aromatics;Heterocycles;Intermediates & Fine Chemicals;Pharmaceutical material and intermeidates;Active Pharmaceutical Ingredients;64228-81-5 | | Mol File: | 64228-81-5.mol | 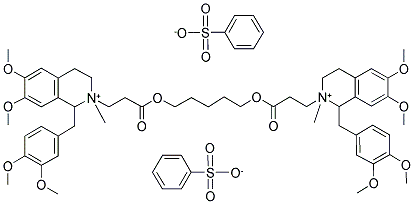 |
| | Atracurium besylate Chemical Properties |
| Melting point | 85-90°C | | storage temp. | Inert atmosphere,2-8°C | | solubility | H2O: ~27 mg/mL | | form | powder | | color | white | | InChIKey | YXSLJKQTIDHPOT-UHFFFAOYSA-N | | CAS DataBase Reference | 64228-81-5(CAS DataBase Reference) |
| Safety Statements | 24/25 | | WGK Germany | 3 | | HS Code | 29334900 |
| | Atracurium besylate Usage And Synthesis |
| Skeletal muscle relaxant for anesthesia |
Atracurium Besilate (Atracurium besylate) is a kind of skeletal muscle relaxant used for general anesthesia; it is suitable for the muscle relaxation required in both the treatment of abdominal surgery and endotracheal intubation. Being same with most neuromuscular blocking agent, it can cause the release of histamine, the occurrence of skin flushing, transient hypotension, and occasional bronchospasm.
Combination between atracurium besilate and inhalation anesthetic gases, such as an alkyl halide, isoflurane and halothane may enhance the neuromuscular blocking effects. Combination with aminoglycoside, polypeptide-class antibiotics, lithium salts, and quinidine or procaine mountain amine may enhance its role in nerve blocking. This product is not suitable to be used in combination with depolarizing muscle relaxants such as suxamethonium which is due to that it may cause prolonged complex blocking effect which is difficult to be reversed by anticholinergic enzyme drugs. Patients who are allergic to this drug should be disabled. It needs to be especially careful for applying this drug in patients of myasthenia gravis, neuromuscular disease, and severe electrolyte imbalance, because it can enhance the effects of other non-depolarizing agents. People suffering from severe cardiovascular disease are more sensitive to the atracurium besilate-induced transient hypotension so it is recommended to apply a slow and graded intravenous injection for administration.
| | Distinguish | At room temperature, atracurium besilate is white-like to yellowish crystalline powder; it is hygroscopic, odorless, easily soluble in chloroform or ethanol, soluble in water, but insoluble in ether.
(1) Take about 10 mg of the product, after adding dilute hydrochloric acid for 1ml to dissolve, add diluted potassium iodide testing solution drop wise, which generates a yellow precipitate.
(2) Take this product, add 0.1mol L hydrochloric acid solution for preparing the solution of concentration being 50μg per 1ml; apply spectrophotometry approach (Chinese Pharmacopoeia 2000 edition of Appendix VI A) for determination with a maximum absorption in the 280nm wavelength.
(3) The infrared absorption spectrum of this product should be consistent with the reference standard map.
The above information is edited by the chemicalbook of Dai xiongfeng.
| | Examination | Color of the solution
Take 100 mg of this product, add 10 ml of water to dissolve it with which the solution should be colorless; if it exhibits some color, compare it with yellow No. 3 colorimetric solution; it should not have a deeper yellow color (Chinese Pharmacopoeia 2000 edition of Appendix IX A first method).
Relative substance
Take a certain amount of this product; add the mobile phase to make a solution of 1mg per 1 mL, as the test solution; take some amount of the accurate standard sample, add the mobile phase for dilution to make a solution of 50 μg per 1 ml as the control solution. Perform according to the specific content and determine under the following chromatographic conditions, take accurately 10 μl of the standard control solution and add into the liquid chromatography; adjust the checking sensitivity and make the height of major component peaks being 20% to 25% of that of full scale; then accurately take 10 μl of the two above solutions and separately inject it into the liquid chromatography, record the test solution chromatograms until reaching 2-fold major component peak retention time. If there exist impure peaks at the chromatogram of the test solution, measure the sum of the amount of every impure peak area; the sum should not be larger than the peak area of the major component of the control solution (5.0%).
Sulfate
Take 1.0 g of this product, add water 30ml to dissolve it, check it according to inspection(Chinese Pharmacopoeia 2000 edition of Appendix VIII B); compare it with the control solution made by 5.0 mL of standard potassium sulfate solution, and should not be thicker (0.05%).
Loss on weight by drying
Take some amount of this product with phosphorus pentoxide as a desiccant, dry under vacuum at 50 °C for 3 hours with the losing weight should not exceed 2.0% (Chinese Pharmacopoeia 2000 edition of Appendix VIII L).
Ignition of residue
Take 1.0 g of this product, check it according to inspection (Chinese Pharmacopoeia 2000 edition of Appendix VIII N), the left residue should not be over 0.1%.
Heavy Metal
Take the residue of ignition and check it according to inspection (Chinese Pharmacopoeia 2000 edition Appendix VIII H method); the heavy metals content should not exceed twenty millionths.
| | Chemical Properties | It is white-like powder; melting point: 85~90 °C. It will be softened at 60 °C.
1R-cis, 1R'cis type (Cisatracurium Besylate): [96946-42-8]. White solid.
| | Uses | 1. It is a kind of non-depolarizing muscle relaxants with the 2.5 times of effect as high as tubocurarine 2.5 times but with a short duration. It is used for a variety of surgical procedures, especially for intubation and caesarean section.
2. It is a skeletal muscle relaxant for general anesthesia.
| | Production methods | Dissolve 0.1mol of 1, 5-pentanediol, 0.2 mol of triethylamine and 0.1g of pyrogallol in 100ml of dry benzene; further add 0.2 mol of acryloyl chloride for dissolution in 60 ml dry benzene solution under vigorous stirring. Then add about 100ml of anhydrous benzene and 10ml of triethylamine, followed by stirring at 50 °C for 0.5h; filter to remove the triethylamine hydrochloride, also remove the solvent under reduced pressure. In the presence of trace amount of p-methoxyphenol, the remaining yellow oil is distilled at darkness; further collect the distillate fraction of 90~95 °C/1.33Pa to obtain 1,5-pentamethylene diacrylate with yield being 61%.
Dissolve 4.43g of tetrahydropapaverine and 1.30 g of the above 1, 5-pentamethylene diacrylate in 15 ml of dry benzene; stir in the darkness and reflux for 48h; further remove the solvent under reduced pressure with the remaining dark red oil being dissolved in 10ml of chloroform. Add about 400ml of diethyl ether; then further add about 500 ml of saturated oxalic acid into ether solution to give flocculent white precipitate. Filter, wash with ether, and dry. Re-crystallize using ethanol twice to give 3.5 g of compound as a white powder (I) in a yield of 51%, m.p. 117~121 °C.
Compound (I) is dissolved in aqueous sodium bicarbonate solution and extracted with toluene. Concentrate the extract to obtain a colorless viscous liquid, which corresponds to compound (II).
0.5g of dry compound (II) is dissolved in 8 ml spectroscopically pure acetonitrile; add methyl benzenesulfonate, and have reaction at room temperature for 22h. Under vigorous stirring, add the solution drop wise into about 450 ml of dry ether. Filter and collected the formed flocculent white precipitates; wash with dry diethyl ether; In the presence of phosphorous pentoxide, dry it in vacuum at 50 °C to obtain white-like atracurium besilate powder with the melting point being 85~90 °C.
| | Chemical Properties | White to yellowish-white powder, slightly hygroscopic | | Originator | Tracrium,Burroughs-Wellcome,US,1983 | | Uses | A neuromuscular blocking agent | | Definition | ChEBI: The bisbenzenesulfonate salt of atracurium. | | Manufacturing Process | Acryloyl chloride (0.2 mol) in dry benzene (60 ml) was added over 0.5 hour
with mechanical stirring to pentane-1,5-diol (0.1 mol), triethylamine (0.2 mol)
and pyrogallol (0.1 g) in dry benzene (100 ml). Further dry benzene (ca 100
ml) was added followed by triethylamine (10 ml), and the mixture stirred at
50°C for 0.5 hour. The triethylamine hydrochloride was filtered off and the
solvent removed in vacuum to leave a yellow oil which was distilled in the
presence of a trace of p-methoxyphenol, excluding light, to give 1,5-
pentamethylenediacrylate (12.9 g, 61%, BP 90° to 95°C/0.01 mm Hg).
A solution of tetrahydropapaverine (4.43 g) and 1,5-pentamethylene
diacrylate (1.30 g) in dry benzene (15 ml) was stirred under reflux for 48
hours excluding light. The solvent was removed in vacuum and the residual
pale red oil dissolved in chloroform (10 ml). Addition of ether (ca 400 ml),
followed by saturated ethereal oxalic acid solution (ca 500 ml) gave a
flocculent white precipitate, which was filtered off, washed with ether and dried. Crystallization (twice) from ethanol gave N,N'-4,10-dioxa-3,11-
dioxotridecylene-1,13-bis-tetrahydropapaverine dioxalate as a white powder
(3.5 g, 51%, MP 117° to 121°C).
The free base, N,N'-14,10-dioxa-3,11-dioxotridecylene-1,13-bistetrahydropapaverine,
was obtained by basifying an aqueous solution of the
dioxalate with sodium bicarbonate solution, followed by extraction with
toluene and evaporation of the solvent, to give a colorless viscous oil.
Scrupulously dried base (0.5 g) in spectroscopically pure acetonitrile (8 ml)
was treated with methyl benzene sulfonate at room temperature for 22 hours.
The filtered reaction mixture was added dropwise to mechanically stirred,
filtered, dry ether (ca 450 ml). The flocculent white precipitate was filtered off,
washed with dry ether, and dried in vacuum over P2O5 at 50°C to yield the
product, an off-white powder melting at 85° to 90°C.
In practice it is usually used as dibenzenesulfonate. | | Brand name | Tracrium (Hospira). | | Therapeutic Function | Neuromuscular blocker | | Biological Functions | Atracurium besylate (Tracrium) is a benzylisoquinolinium
compound like d-tubocurarine. Its actions are similar
to those of d-tubocurarine, but its duration of action
is shorter (45 minutes) because of spontaneous degradation
of the molecule (Hofmann elimination). Because
of this, atracurium is useful in patients with low or atypical
plasma cholinesterase and in patients with renal or
hepatic impairment. | | General Description | Atracurium besylate, 2-(2-carboxyethyl)-1,2,3,4-tetrahydro-6,7-dimethoxy-2-methyl-1-veratrylisoquinolinium benzenesulfonate pentamethyleneester (Tracrium), is a nondepolarizing neuromuscular blockingagent that is approximately 2.5 times more potent thand-tubocurarine. Its duration of action (half-life, 0.33 hours)is much shorter than that of d-tubocurarine. | | Biochem/physiol Actions | Nicotinic acetylcholine receptor antagonist. | | Clinical Use | Atracurium Besylate is metabolized rapidly and nonenzymatically to yield laudanosineand a smaller quaternary compound ,which do not have neuromuscular blocking activity. In vitroexperiments show that atracurium besylate breaks down atpH 7.4 and 37°C by a Hoffman elimination reaction.Atracurium besylate undergoes enzymatic decompositionof its ester function to yield an inactive quaternary alcoholand quaternary acid. AChE inhibitors such as neostigmine,edrophonium, and pyridostigmine antagonize paralysis byatracurium besylate. | | Veterinary Drugs and Treatments | Atracurium is indicated as an adjunct to general anesthesia to
produce muscle relaxation during surgical procedures or mechanical
ventilation and also to facilitate endotracheal intubation.
Atracurium can be used in patients with significant renal or hepatic
disease. | | Drug interactions | Potentially hazardous interactions with other drugs
Anaesthetics: effects enhanced by ketamine;
enhanced effect with volatile liquid general
anaesthetics.
Anti-arrhythmics: procainamide enhances muscle
relaxant effect.
Antibacterials: aminoglycosides, clindamycin,
polymyxin and piperacillin enhance effect of
atracurium.
Antiepileptics: muscle relaxant effects antagonised by
carbamazepine; effects reduced by long-term use of
phenytoin but might be increased by acute use.
Atracurium enhances the neuromuscular block
produced by botulinum toxin (risk of toxicity) | | Metabolism | Atracurium besilate undergoes spontaneous degradation
via Hofmann elimination (a non-enzymatic breakdown
process occurring at physiological pH and temperature)
to produce laudanosine and other metabolites. There is
also ester hydrolysis by non-specific plasma esterases.
The metabolites have no neuromuscular blocking activity.
Excretion of atracurium is in urine and bile, mostly as
metabolites. |
| | Atracurium besylate Preparation Products And Raw materials |
| Raw materials | 6-Benzylaminopurine-->Triethylamine hydrochloride-->Pyrogallol-->Acryloyl chloride-->1,5-Pentanediol-->PAPAVERINE HYDROCHLORIDE-->Methyl benzenesulfonate-->Salmeterol xinafoate-->(+/-)-Tetrahydropapaverine-->(1R,2R,3aS,9aS)-2,3,3a,4,9,9a-Hexahydro-1-[(3S)-3-hydroxyoctyl]-1H-benz[f]indene-2,5-diol-->1,5-DIAMINOPENTANE DIHYDROCHLORIDE |
|