|
ChemicalBook Optimization Suppliers |
|
| 化学名: | クエン酸鉄アンモニウム | | 英語化学名: | Ammonium ferric citrate | | 别名: | AMMONIUM FERRIC CITRATE, BROWN, 20.5-22. 5%;AMMONIUM IRON(III) CITRATE GREEN, GRANUL ATED, N. F. XI;AMMONIUM IRON(III) CITRATE RED BROWNN. &;Ferric Ammonium Citrate, Green Powder;AmmoniumFerricCitrateUsp;Ammonium iron(III) citrate, 16.5 to 18.5% Fe;FERRIC AMMONIUM CITRATE PURIFIED;FERRICAMMONIUMCITRATE,BROWN,POWDER,FCC | | CAS番号: | 1185-57-5 | | 分子式: | C6H11FeNO7 | | 分子量: | 265 | | EINECS: | 214-686-6 | | カテゴリ情報: | Organic-metal salt | | Mol File: | 1185-57-5.mol | 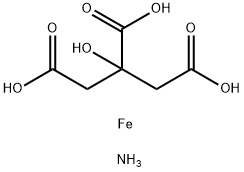 |
| 融点 | >193°C (dec.) | | 沸点 | 498℃[at 101 325 Pa] | | 比重(密度) | 1.06[at 20℃] | | 蒸気圧 | 0Pa at 25℃ | | 貯蔵温度 | Store at RT. | | 溶解性 | 1200g/l | | 外見 | powder | | 色 | Green | | 臭い (Odor) | Odorless to slight ammonia odor | | PH | 6-8 (100g/l, H2O, 20℃) | | 水溶解度 | 1200 g/L (20 ºC) | | Sensitive | Hygroscopic | | Merck | 14,4017 | | 暴露限界値 | ACGIH: TWA 1 mg/m3
NIOSH: TWA 1 mg/m3 | | 安定性: | Hygroscopic | | LogP | -0.737 at 25℃ | | CAS データベース | 1185-57-5(CAS DataBase Reference) | | EPAの化学物質情報 | Ferric ammonium citrate (1185-57-5) |
| | クエン酸鉄アンモニウム Usage And Synthesis |
| 外観 | 緑色、赤褐色、深赤色、褐色又は帯褐黄色で、透明なりん片状結晶、粉末、粒又は塊 | | 溶解性 | 水に易溶。エタノールに不溶。水に極めて溶けやすく、エタノールにほとんど溶けない。 | | 解説 | クエン酸鉄アンモニウム,クエン酸の第二鉄塩。鉄分補給として貧血用薬などに含有。2価および3価の鉄塩の存在が知られているが,2価のものはきわめて不安定である。3価の塩Fe(NH4)2H(C6H5O7)2はクエン酸鉄(III)にアンモニアを作用させると生成する。 | | 用途 | 医薬品原料、鉄及び補血強壮剤研究用。 | | 用途 | 食品の鉄強化剤、医薬品原料、青写真材料。 | | 効能 | 診断補助薬 (造影剤) | | 商品名 | フェリセルツ (大塚製薬) | | 使用上の注意 | 空気中で湿気を吸収しやすく、光によって変質する。 | | 説明 | Ammonium ferric citrate is a food additive with E number E381 used as an acidity regulator. It is a green or reddish-brown powder which is very soluble in water.
The molecular formula of ammonium iron (III) citrate is variable. It can be prepared by adding Fe(OH)3 to an aqueous solution of citric acid and ammonia . The brown form is approximately 9% NH3, 16.5 – 18.5 % Fe , and 65 % hydrated citric acid; the green form is approximately 7.5 % NH3 , 14.5 – 16 % Fe, and 75% hydrated citric acid. The green type is more readily reduced by light than the brown.
Other uses for ammonium ferric citrate include water purification and printing (cyano type). It is used as a reducing agent of metal salts of low activity like gold and silver and is also in a commonly used recipe with potassium ferricyanide to make cyanotype prints. Ammonium ferric citrate is also used in Kligler iron deeps to determine hydrogen sulfide production in microbial metabolism.
In medicine, ammonium ferric citrate is used as a contrast medium. It is also used as a hematinic. | | 化学的特性 | brownish-yellow to brown powder | | 化学的特性 | Ferric ammonium citrate forms reddish brown
flakes or grains, or a brownish-yellow powder. It has a
slight ammonia odor. There is also a green form that is
odorless. | | 化学的特性 | Ferric ammonium citrate (iron (III) ammonium citrate) is prepared by the reaction of ferric hydroxide with citric acid, followed by treatment with ammonium hydroxide, evaporating and drying. The resulting product occurs in two forms depending on the stoichi- ometry of the initial reactants. (1) Ferric ammonium citrate (iron (HI) ammonium citrate, CAS No. 1332-98-5) is a complex salt of undetermined structure composed of 16.5 to 18.5% iron, approximately 9% ammonia, and 65% citric acid and occurs as reddish brown or garnet red scales or granules or as a brownish-yellowish powder. (2) Ferric ammonium citrate (iron (HI) ammonium citrate, CAS No. 1333-00-2) is a complex salt of undetermined structure composed of 14.5 to 16% iron, approximately 7.5% ammonia and 75% citric acid and occurs as thin transparent green scales, as granules, as a powder, or as transparent green crystals. Supposedly, ferric ammonium citrate is one of the few soluble iron compounds which can be added to dairy products without inducing off-flavors. | | 使用 | Iron Ammonium Citrate is an anticaking agent used in salt. It is the chemical green ferric ammonium citrate. | | 使用 | Ferric Ammonium Citrate is a nutrient supplement that is a source of iron. It is prepared by the reaction of ferric hydroxide with citric acid, followed by treatment with ammonium hydroxide, evaporation, and drying. The resulting product occurs in two forms, i.e., brown with 16.5–18.5% iron and ferric ammonium citrate, green with 14.5–16% iron. | | 使用 | For blueprints; in photography. | | 使用 | Hematinic | | 一般的な説明 | Ammonium ferric citrate is a yellowish brown to red solid with a faint odor of ammonia. Ammonium ferric citrate is soluble in water. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. Ammonium ferric citrate is used in medicine, in making blueprints, and as a feed additive. | | 空気と水の反応 | Water soluble. | | 反応プロフィール | Acidic salts, such as Ammonium ferric citrate, are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions. Special Hazards of Combustion Products: Toxic oxides of nitrogen or ammonia gas may be formed in fires [USCG, 1999]. | | 健康ハザード | Inhalation of dust irritates nose and throat. Ingestion causes irritation of mouth and stomach. Dust irritates eyes and causes mild irritation of skin on prolonged contact. | | 火災危険 | Special Hazards of Combustion Products: Toxic oxides of nitrogen or ammonia gas may be formed in fires. | | 燃焼性と爆発性 | Not classified | | 職業ばく露 | Ferric ammonium citrate is used in
blueprinting, photography, medical treatment; and as an
animal food additive. | | 応急処置 | If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit. Thesymptoms of metal fume fever may be delayed for 4-12 hfollowing exposure: it may last less than 36 h.Note to physician: In case of fume inhalation, treat pneumonitis. Give prednisone or other corticosteroid orally toreduce tissue response to fume. Positive-pressure ventilationmay be necessary. Treat metal fume fever with bed rest,analgesics, and antipyretics.Note to physician: For severe poisoning do not use BAL[British Anti-Lewisite, dimercaprol, dithiopropanol(C3H8OS2)] as it is contraindicated or ineffective in poisoning from iron. | | 貯蔵 | Color Code—Green: General storage may be used.Prior to working with this chemical you should be trainedon its proper handling and storage. Store in tightly closedcontainers in a cool, well-ventilated area away from light.Sources of ignition, such as smoking and open flames, areprohibited where ferric ammonium citrate is used, handled,or stored in a manner that could create a potential fire orexplosion hazard. | | 輸送方法 | UN3077 Environmentally hazardous substances,
solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required. | | 不和合性 | Compounds of the carboxyl group react
with all bases, both inorganic and organic (i.e., amines)
releasing substantial heat, water and a salt that may be
harmful. Incompatible with arsenic compounds (releases
hydrogen cyanide gas), diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides
(releasing heat, toxic and possibly flammable gases), thiosulfates and dithionites (releasing hydrogen sulfate and oxides of sulfur). |
|