- PIERICIDIN A
-

- $1.00 / 1g
-
2019-12-26
- CAS:2738-64-9
- Min. Order: 1g
- Purity: ≥98%
- Supply Ability: g/kg/Ton
|
| | PIERICIDIN A Basic information |
| Product Name: | PIERICIDIN A | | Synonyms: | ,5,7,11-tetramethyl-,(all-e)-(4s,5s)-;2,6,9,11-tridecatetraen-4-ol,13-(4-hydroxy-5,6-dimethoxy-3-methyl-2-pyridyl)-3;piericidin;piericidin(sub1);piericidinea;SN 198E;SHAOGUAMYCIN B;SHAOGUANMYCIN B | | CAS: | 2738-64-9 | | MF: | C25H37NO4 | | MW: | 415.57 | | EINECS: | | | Product Categories: | Antibiotic | | Mol File: | 2738-64-9.mol | 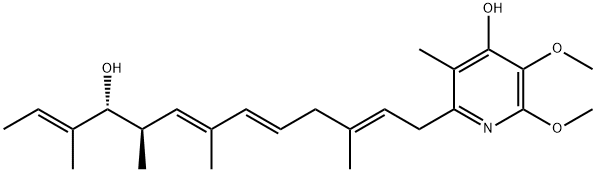 |
| | PIERICIDIN A Chemical Properties |
| Boiling point | 614.9±55.0 °C(Predicted) | | density | 1.044±0.06 g/cm3(Predicted) | | Fp | 85 °C | | storage temp. | 2-8°C | | solubility | Soluble in DMSO (up to 25 mg/ml) or in Ethanol (up to 20 mg/ml). | | pka | 5.21±0.33(Predicted) | | form | liquid | | color | green-yellow | | Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO or Ethanol may be stored at -20°C for up to 1 month. |
| | PIERICIDIN A Usage And Synthesis |
| Description | Piericidin A (2738-64-9) is a potent inhibitor of the mitochondrial and bacterial type I NADH-ubiquinone oxidoreductase (complex I).1?Piericidin A is a ubiquinone analog which binds to the ubiquinone binding site of the enzyme.2?It is an extremely useful tool for exploring the role of complex I in mitochondrial function in both normal and pathophysiology.3-5?Prevents upregulation of GRP78 and induces cell death in glucose-deprived, etoposide-resistant HT-29 cells (IC50=7.7 nM).6 | | Uses | Piericidin A is the major analogue of a family of pyridyl antibiotics isolated from selected Streptomyces species. It is a specific, potent inhibitor of NADH-ubiquinone oxidoreductase (Complex I) that binds to ubiquinone binding site(s). Piericidin A inhibits both mitochondrial and bacterial NADH-ubiquinone oxidoreductases, binding close to NUOD-NUOB interface. | | Uses | Piericidin A is an antibiotic and a member of the Class I inhibitors that acts as an effective inhibitor of Complex I. | | Definition | ChEBI: Piericidin A is a member of the class of monohydroxypyridines that acts as an irreversible mitochondrial Complex I inhibitor that strongly associates with ubiquinone binding sites in both mitochondrial and bacterial forms of NADH:ubiquinone oxidoreductase It has a role as a mitochondrial respiratory-chain inhibitor, an EC 1.6.5.3 [NADH:ubiquinone reductase (H(+)-translocating)] inhibitor, an antimicrobial agent and a bacterial metabolite. It is a monohydroxypyridine, a member of methylpyridines, an aromatic ether and a secondary allylic alcohol. | | Biochem/physiol Actions | Piericidin A (PA), an insecticidal metabolite of Streptomyces mobaraensis is a potential quorum-sensing inhibitors (QSIs) that can destroy the expression of the virulence genes of Erwinia carotovora subsp. atroseptica (Eca). Hence it may be used as control agents of soft rot disease on potato tubers. | | in vitro | previous study found that piericidin a could inhibit the electron transport system at two sites. piericidin a specifically reacted at very low concentrations at a site near the reduced nadh dehydrogenase. at high concentrations, piericidin a inhibited the succinic dehydrogenase system, and the inhibition could be partially reversed by coenzyme q. moreover, it was found that both the succinate and nadh oxidase system were inhibited at high levels of piericidin a [1]. | | in vivo | animal study was performed to evaluate the effect of piericidin a and antagonistic effect of vitamin k3 on respiration, blood pressure and heart rate in rats. results showed that 0.167 mg/kg of piericidin a increased the respiratory rate and the lowered blood pressure rapidly. furthermore, vitamin k3 (10-40 mg/kg) could restore the responses to piericidin a in rats [2]. | | Enzyme inhibitor | This reduced antibiotic (FW = 423.64 g/mol; CAS 2738-64-9; Soluble in ethanol, methanol, DMF or DMSO) is a structural analogue of ubiquinone and a partially competitive inhibitor of bacterial and mitochondrial Type-I NADH-ubiquinone oxidoreductases (or Complex I). Octahydropiericidin A also inhibits glucose dehydrogenase. | | References | 1) Fato?et al.?(2009),?Differential effects of mitochondrial Complex I inhibitors on production of reactive oxygen species; Biochim. Biophys. Acta,?1787?384
2) Zhou and Fenical (2016),?The unique chemistry and biology of the piericidins; J. Antibiot. (Tokyo),?69?582
3) Bongard?et al.?(2015),?The effects of mitochondrial complex I blockade on ATP and permeability in rat pulmonary microvascular endothelial cells in culture (PMVEC) are overcome by coenzyme Q1 (CoQ1); Free Radic. Biol. Med.,?79?69
4) Lee?et al.?(2013),?Isoniazid-induced cell death is precipitated by underlying mitochondrial complex I dysfunction in mouse hepatocytes; Free Radic. Biol. Med.,?65?584
5) Choi?et al.?(2011),?Loss of mitochondrial complex I activity potentiates dopamine neuron death induced by microtubule dysfunction in a parkinson’s disease model; J. Cell Biol.,?192?873
6) Hwang?et al.?(2008),?Etoposide-resistant HT-29 human colon carcinoma cells during glucose deprivation are sensitive to piericidin A, a GRP78 down regulator; J. Cell Physiol.,?215?243 |
| | PIERICIDIN A Preparation Products And Raw materials |
|