|
|
| | amicarbalide Basic information |
| Product Name: | amicarbalide | | Synonyms: | amicarbalide;3,3'-(Carbonyldiimino)bis(benzenecarbimidamide);3,3'-Carbonyldiiminobis(benzenecarboxamidine);3,3'-Ureylenebisbenzamidine;M&B 5062 A;1,3-bis(3-amidinophenyl)urea;1,3-bis(3-carbamimidoylphenyl)urea;3,3'-Diamidinocarbanilide | | CAS: | 3459-96-9 | | MF: | C15H16N6O | | MW: | 296.332 | | EINECS: | 2224027 | | Product Categories: | | | Mol File: | 3459-96-9.mol | 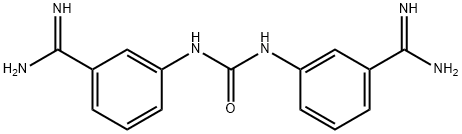 |
| | amicarbalide Chemical Properties |
| density | 1.40 | | pka | 13.69±0.70(Predicted) |
| | amicarbalide Usage And Synthesis |
| Originator | Diampron,May and Baker | | Manufacturing Process | m-Aminobenzonitrile (50 g) in anhydrous pyridine (200 ml) was treated with a
solution of phosgene (15 ml) in anhydrous toluene (100 ml) over 10 min with
mechanical stirring. The red solution was heated for 0.5 hour on the steam
bath, cooled, and added to water (2 litres). The pale grey precipitate was
filtered off, washed with ether, and crystallised from methanol (250 ml). N,N1-
Di(m-cyanophenyl)urea separated as grey prisms, melting point 205-206°C.
A suspension of N,N1-di(m-cyanophenyl)urea (42 g) in anhydrous chloroform
(420 ml), containing anhydrous ethanol (70 ml), was saturated with
anhydrous hydrogen chloride at 0-5°C. After setting aside for 2 hours, a clear
solution was obtained, which began to crystallise. After a week, the crystals
were filtered off, washed well with anhydrous ether, and dried over calcium
chloride. The iminoether hydrochloride so obtained (72 g) was added to
saturated anhydrous ethanolic ammonia (720 ml), and the suspension heated
at 55-60°C for 6 hours. After cooling to 20°C the crystals were filtered off,
and recrystallized from 2 N hydrochloric add (300 ml). 3,31-
Diamidinodiphenylurea dihydrochloride monohydrate separated as white
prisms, melting point 286°C (decomp.).
In practice it is usually used as isethonate salt. | | Therapeutic Function | Antiprotozoal |
| | amicarbalide Preparation Products And Raw materials |
|