
Magnesium carbonate
| Price | $1 |
| Package | 1KG |
| Min. Order: | 1KG |
| Supply Ability: | 100kg |
| Update Time: | 2019-12-31 |
Product Details
| Product Name: Magnesium carbonate | CAS No.: 13717-00-5 |
| Min. Order: 1KG | Purity: 99% |
| Supply Ability: 100kg | Release date: 2019/12/31 |
| name: Cassie446 |
▼
▲
Product Name:
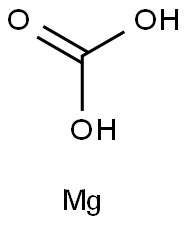
Magnesium carbonate
Synonyms:
MAGNESIUM CARBONATE BASIC HYDRATE;MAGNESIUM CARBONATE;MAGNESITE;MAGNESIA 81010;MAGNESIA 81811;MAGGRAN(R) MC;MAGGRAN(R) MCPLUS;MAGNESIUM CARBONATE HYDRATE
CAS:
13717-00-5
MF:
CMgO3
MW:
84.31
EINECS:
208-915-9
Product Categories:
-
Mol File:
13717-00-5.mol
▼
▲
Magnesium carbonate Chemical Properties
▼
▲
Melting point
2200 °C
density
3.001 g/cm3
Solubility Product Constant (Ksp)
pKsp: 5.17
CAS DataBase Reference
13717-00-5(CAS DataBase Reference)
EPA Substance Registry System
Magnesite (13717-00-5)
▼
▲
Safety Information
▼
▲
▼
▲
MSDS Information
▼
▲
Provider
Language
ACROS
English
ALFA
English
▼
▲
Magnesium carbonate Usage And Synthesis
▼
▲
Chemical Properties
Magnesium carbonate, MgC03, is a light, bulky white powder, very slightly soluble in water,loses CO2 even on gentle heating. It is used extensively in the manufacture of food products, pharmaceuticals, cosmetics, and magnesium salts.
Occurrence and Uses
Magnesium carbonate occurs in nature in several minerals as hydrated, basic and double salts, as shown above. The two principal minerals are magnesite, MgCO3 and dolomite, a double salt, CaCO3•MgCO3. Both minerals are used as source materials in the production of magnesium metal. Also, they are calcined to produce basic refractory bricks. Other applications of magnesium carbonate are in flooring, fireproofing and fire-extinguishing compositions; as a filler material and smoke suppressant in plastics; as a reinforcing agent in neoprene rubber; as a drying agent and for color retention in foods; in cosmetics; in dusting powder; and in toothpaste. The high purity magnesium carbonate is used as an antacid in medicine; and as an additive to table salt. Another important application of magnesium carbonate is as a starting material in producing a number of magnesium compounds.
Preparation
Magnesium carbonate is obtained mainly by mining its natural mineral magnesite. The trihydrate salt, MgCO3•3H2O, is prepared by mixing solutions of magnesium and carbonate ions in the presence of carbon dioxide. Alternatively, it may be produced by carbonation of a magnesium hydroxide slurry with carbon dioxide under pressure (3.5 to 5 atm) and at a temperature below 50°C which yields soluble magnesium bicarbonate:
Mg(OH)2 + 2CO2 → Mg(HCO3)2
The solution is filtered to remove impurities and the filtrate is subjected to vacuum or aeration to yield insoluble magnesium carbonate as a hydrated salt:
Mg2 + 2HCO3¯ → MgCO3 + CO2 + H2O
Under ordinary conditions, anhydrous magnesium carbonate cannot be prepared in aqueous systems. The anhydrous salt, however, can be made under very high partial pressures of carbon dioxide.
Basic magnesium carbonate occurs in nature as the mineral hydromagnesite. The basic salt is obtained by mining the ore followed by purification. The basic carbonates also can be made by drying the magnesium carbonate trihydrate at about 100°C. Alternatively it can be prepared by simply boiling a solution of magnesium bicarbonate. The bicarbonate is obtained by carbonation of a magnesium hydroxide slurry below 50°C and under a CO2 partial pressure of 3.5 to 5 atm. Composition of the basic carbonate produced by the above methods is 4MgCO3 •Mg(OH)2•4H2O.
Another basic salt, MgCO3•Mg(OH)3•3H2O is precipitated when magnesium salt solution is treated with sodium carbonate solution. The reactions probably are:
CO32– + H2O → HCO3¯ + OH¯
2Mg2+ + CO32– + 2OH¯ → MgCO3•Mg(OH)2
Mg(OH)2 + 2CO2 → Mg(HCO3)2
The solution is filtered to remove impurities and the filtrate is subjected to vacuum or aeration to yield insoluble magnesium carbonate as a hydrated salt:
Mg2 + 2HCO3¯ → MgCO3 + CO2 + H2O
Under ordinary conditions, anhydrous magnesium carbonate cannot be prepared in aqueous systems. The anhydrous salt, however, can be made under very high partial pressures of carbon dioxide.
Basic magnesium carbonate occurs in nature as the mineral hydromagnesite. The basic salt is obtained by mining the ore followed by purification. The basic carbonates also can be made by drying the magnesium carbonate trihydrate at about 100°C. Alternatively it can be prepared by simply boiling a solution of magnesium bicarbonate. The bicarbonate is obtained by carbonation of a magnesium hydroxide slurry below 50°C and under a CO2 partial pressure of 3.5 to 5 atm. Composition of the basic carbonate produced by the above methods is 4MgCO3 •Mg(OH)2•4H2O.
Another basic salt, MgCO3•Mg(OH)3•3H2O is precipitated when magnesium salt solution is treated with sodium carbonate solution. The reactions probably are:
CO32– + H2O → HCO3¯ + OH¯
2Mg2+ + CO32– + 2OH¯ → MgCO3•Mg(OH)2
Description
Magnesium carbonate has the molecular formula of MgCO3 and the molecular weight of 84.3145 g/mol.For the most part,Mg2+ forms several hydrated and basic carbonates that are stable and occur in nature.
The calcite structure of magnesium carbonate has the form wherein Mg2+ is surrounded by six O2- atoms. The dihydrate composition has a triclinic structure, while the trihydrate has a monoclinic structure. References to “light” and “heavy” magnesium carbonates actually refer to the magnesium hydroxycarbonates. A space-filling structure of the anhydrous salt is seen in the above diagram where the triangular CO3 2- groups are clearly visible.
The calcite structure of magnesium carbonate has the form wherein Mg2+ is surrounded by six O2- atoms. The dihydrate composition has a triclinic structure, while the trihydrate has a monoclinic structure. References to “light” and “heavy” magnesium carbonates actually refer to the magnesium hydroxycarbonates. A space-filling structure of the anhydrous salt is seen in the above diagram where the triangular CO3 2- groups are clearly visible.
Chemical Properties
Light, bulky, white powder. Soluble in acids; very slightly soluble in water; insoluble in alcohol. Noncombustible.
Uses
Magnesium Carbonate is an anticaking agent and general purpose food additive. it is practically insoluble in water but is more soluble in water containing carbon dioxide. it imparts a slightly alkaline reaction to the water. it is used as an alkali in sour cream, butter, and canned peas. it is used as an anticaking agent in table salt and dry mixes. it assists in providing clarity in algin gels and functions as a filler in dental impression materials.
Uses
Magnesium salts, heat insulation and refractory, rubber reinforcing agent, inks, glass, pharmaceuticals, dentifrice and cosmetics, free-running table salts, antacid, making magnesium citrate, filtering medium. In foods as drying agent, color retention agent, anticaking agent, carrier.
Uses
Magnesium carbonate (MgCO3) is found in a mixture of natural minerals. It can also be produced in several ways, including pumping carbon dioxide through magnesium oxide or magnesium hydroxide. It is used in pharmaceuticals such as magnesium citrate and as a desiccant to keep hydroscopic products from caking (table salt) and to strengthen rubber and produce dyes, inks, and cosmetics.
Definition
The term magnesium carbonate is generally reserved for the synthetic, pure variety. The naturally occurring material is called magnesite.
▼
▲
Magnesium carbonate Preparation Products And Raw materials
▼
▲
Raw materials
Sodium carbonate-->Magnesium chloride hexahydrate-->Magnesium chloride-->Dolomite (CaMg(CO3)2), dead-burned refractory
Preparation Products
Acrylonitrile-->Magnesium oxide-->Magnesium sulfate-->POTASSIUM BICARBONATE-->Magnesium chloride hexahydrate-->Magnesium chloride-->Magnesium hydroxide-->Amino resin paint-->Fiber Glass Wool-->2-Amino-4,8-naphthalenedisulfonic acid-->1-Aminonaphthalene-6-sulfonic acid-->5-Amino-1-naphthalenesulfonic acid-->Magnesium fluorosilicate-->Magnesium fluosilicate-->magnesium carbonate for medicanaln-->Magnesium nitrate hexahydrate-->MAGNESIUM GLUCONATE-->MAGNESIUM HYDROGEN ORTHOPHOSPHATE-->MAGNESIUM L-GLUTAMATE TETRAHYDRATE-->Magnesium fluoride-->Magnesium acetate tetrahydrate
Company Profile Introduction
Established in 2014,Career Henan Chemical Co. is a manufacturerspecializing in the sale of fine chemicals.
Mainly deals in the sales of:
Pharmaceutical intermediates
OLED intermediates:
Pharmaceutical intermediates;
OLED intermediates;
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $35.00/1kg |
VIP1Y
|
Hebei Zhuanglai Chemical Trading Co.,Ltd
|
2024-05-29 | |
| $50.00/1kg |
VIP1Y
|
Ouhuang Engineering Materials (Hubei) Co., Ltd
|
2024-04-19 | |
| $0.00/1Kg |
VIP1Y
|
Shaanxi TNJONE Pharmaceutical Co., Ltd
|
2024-04-09 | |
| $0.00/1kg |
VIP4Y
|
Hebei Yanxi Chemical Co., Ltd.
|
2023-09-05 | |
| $0.00/25KG |
VIP4Y
|
Hebei Mojin Biotechnology Co., Ltd
|
2023-06-20 | |
| $0.00/1KG |
VIP2Y
|
Hebei Guanlang Biotechnology Co., Ltd.
|
2023-01-11 | |
| $1.00/1kg |
VIP2Y
|
Hebei Duling International Trade Co. LTD
|
2022-12-20 | |
| $15.00/1KG |
Zhuozhou Wenxi import and Export Co., Ltd
|
2021-06-27 | ||
| $1.00/1PCS |
VIP6Y
|
Hebei Guanlang Biotechnology Co., Ltd.
|
2021-02-01 | |
| $10.00/25kg |
VIP7Y
|
Jiangsu Kolod Food Ingredients Co.,Ltd.
|
2018-12-14 |
- Since: 2014-12-17
- Address: Room 702, Floor 7, Building 10, National University Science Park, High-Tech Zone, Zhengzhou City, H
INQUIRY





 China
China