
Calcium cyanamide NEW
| Price | Get Latest Price |
| Package | 25KG |
| Min. Order: | 1KG |
| Supply Ability: | 50000KG/month |
| Update Time: | 2023-08-18 |
Product Details
| Product Name: Calcium cyanamide | CAS No.: 156-62-7 |
| EC-No.: 205-861-8 | Min. Order: 1KG |
| Purity: 99% | Supply Ability: 50000KG/month |
| Release date: 2023/08/18 |
| CAS: | 156-62-7 |
| MF: | CCaN2 |
| MW: | 80.1 |
| EINECS: | 205-861-8 |
| Product Categories: | Inorganics;Pharmaceutical Intermediates |
| Mol File: | 156-62-7.mol |
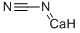 | |
| Calcium cyanamide Chemical Properties |
| Melting point | >300 °C(lit.) |
| density | 2.29 |
| vapor pressure | 0.51Pa at 20℃ |
| form | Powder |
| color | Gray to dark gray |
| Specific Gravity | 2.29 |
| Water Solubility | insoluble H2O, but undergoes hydrolysis releasing acetylene and ammonia [HAW93] [MER06] |
| Sensitive | Moisture Sensitive |
| Merck | 14,1662 |
| BRN | 4124391 |
| InChIKey | QFSRQFUIHVTIDL-UHFFFAOYSA-N |
| LogP | -0.72 at 20℃ |
| CAS DataBase Reference | 156-62-7(CAS DataBase Reference) |
| EPA Substance Registry System | Calcium cyanamide (156-62-7) |
| Calcium cyanamide Usage And Synthesis |
| Chemical Properties | Calcium cyanamide is a blackish-gray, shiny crystalline material or powder. |
| Physical properties | Pure product is a colorless, hexagonal crystal or white powder. Commercial grade material may be grayish-black powder or lump (the color is due to presence of calcium carbide and other impurities); density 2.29 g/cm3; melts around 1,340°C; sublimes around 1,150 to 1,200°C on rapid heating; reacts with water. |
| Uses | Manufacture of calcium cyanide and dicyandiamide; formerly used as a defoliant and herbicide |
| Uses | Calcium Cyanamide is used as a fertilizer, herbicide, insecticide, a steel-making additive and an ore processing material. It can also be used to make thiourea, guanidine and ferrocyanides. manufacture of calcium cyanide, melamine, dicyandiamide. |
| Uses | Calcium cyanamide has its major use as a fertilizer. However, it has a number of other uses, such as a herbicide and a defoliant for cotton plants. It is finding increasing use as a chemical intermediate. For example, it is being used to produce dicyandiamide, which in turn can be polymerized to form the widely used monomer, melamine. The conversion to calcium cyanide and hence into a variety of other uses is also important commercially. |
| Definition | ChEBI: The calcium salt of cyanamide, formed when calcium carbide reacts with nitrogen |
| Definition | calcium cyanamide: A colourlesssolid, CaCN2, which sublimes at1300°C. It is prepared by heating calciumdicarbide at 800°C in a streamof nitrogen: CaC2(s) + N2(g) → CaCN2(s) + C(s) The reaction has been used as amethod of fixing nitrogen in countriesin which cheap electricity isavailable to make the calcium dicarbide(the cyanamide process). Calciumcyanamide can be used as afertilizer because it reacts with waterto give ammonia and calcium carbonate: CaCN2(s) + 3H2O(l) → CaCO3(s) +2NH3(g) It is also used in the production ofmelamine, urea, and certain cyanidesalts. |
| Production Methods | Calcium cyanamide was first produced commercially around 1900 as a fertilizer. The process of making calcium cyanamide involves three raw materials—coke, coal, and limestone— plus nitrogen. The limestone (calcium carbonate) is burned with coal to produce calcium oxide. The calcium oxide is then allowed to react with amorphous carbon in the furnace at 2000°C with the formation of calcium carbide (CaC2). Finely powdered calcium carbide is heated to 1000°C in an electric furnace into which pure nitrogen is passed. It is then removed and uncombined calcium carbide removed by leaching. |
| Preparation | Calcium cyanamide is prepared from calcium carbide. The carbide powder is heated at about 1,000°C in an electric furnace into which nitrogen is passed for several hours. The product is cooled to ambient temperatures and any unreacted carbide is leached out cautiously with water. CaC2 + N2 → CaCN2 + C (ΔHƒ°= –69.0 kcal/mol at 25°C) |
| General Description | A colorless to gray, odorless solid. May cause illness from ingestion. May irritate the skin. If exposed to water or high temperatures, calcium cyanamide may generate toxic and flammable fumes. Used to make pesticides and in fertilizers. |
| Air & Water Reactions | Depending on the calcium carbide content, the cyanamide reacts with water (moisture from air or soil) to produce acetylene and hydrated calcium oxide or calcium hydroxide. Absorption of water during handling or storage of technical calcium cyanamide may cause explosion [Pieri, M. Chem. Abs. 46, 8335 1952]. |
| Reactivity Profile | When hydrated CALCIUM CARBIDE generates salts of calcium that are basic and are generally soluble in water. The resulting solutions contain moderate concentrations of hydroxide ions and have pH's greater than 7.0. They react as bases to neutralize acids. These neutralizations generate heat, but less or far less than is generated by neutralization of the bases in reactivity group 10 (Bases) and the neutralization of amines. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. |
| Hazard | Fire risk with moisture or combined with calcium carbide. Skin, eye, and upper respiratory tract irritant. Questionable carcinogen. |
| Health Hazard | Inhalation or contact with vapors, substance or decomposition products may cause severe injury or death. May produce corrosive solutions on contact with water. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control may cause pollution. |
| Fire Hazard | Produce flammable gases on contact with water. May ignite on contact with water or moist air. Some react vigorously or explosively on contact with water. May be ignited by heat, sparks or flames. May re-ignite after fire is extinguished. Some are transported in highly flammable liquids. Runoff may create fire or explosion hazard. |
| Flammability and Explosibility | Nonflammable |
| Agricultural Uses | Calcium cyanamide (CaCN2) is a dark colored, granulated material containing around 21 % nitrogen. Its dark color is due to the presence of calcium carbide. Calcium cyanamide is produced by heating a mixture of limestone with coal in a nitrogen atmosphere. Generally, the process is carried out in three steps. In the first step, calcium carbonate (limestone) is decomposed at about 1100°C. In the second step, calcium oxide (CaO) and coke (or coal) are heated in an electric furnace to produce calcium carbide. The final step involves heating the powdered calcium carbide at about 1100°C with pure nitrogen (produced by liquefaction of air and fractional distillation) to produce calcium cyanamide. The fertilizer-grade calcium cyanamide contains 21 % nitrogen, 11 % calcium, 11 % free carbon, 5% oil, 2 to 4% water and oxides of aluminum, iron and silicon. In the presence of moisture and air, calcium dicyandiamide (a poisonous compound) is formed. It distinctly leaves alkalinity in the soil equivalent to 1.3 kg calcium carbonate (CaCO3) per 0.45 kg of nitrogen applied. At pH 7 or below, calcium dicyandiamide is converted into urea and lime within one week of its being in the soil. When dry, calcium cyanamide is dusty but it is generally used as granules. It is poisonous, irritating to the skin and used as a pesticide, fertilizer and defoliant in cotton. It is as good a fertilizer as sodium nitrate or ammonium sulphate, but not as fast acting. Calcium cyanamide is an excellent weed killer, especially for tobacco plants, when applied 2 to 3 weeks before sowing. It is also used for producing melamine, urea and certain cyanide salts. |
| Safety Profile | Poison by ingestion, inhalation, sh contact, intravenous, and intraperitoneal routes. Moderately toxic to humans by ingestion. Questionable carcinogen with experimental tumorigenic data. Mutation data reported. The fatal dose, by ingestion, is probably around 20 to 30 g for an adult. It does not have a cyanide effect. Calcium cyanamide is not believed to have a cumulative action. Flammable. Reaction with water forms the explosive acetylene gas. When heated to decomposition it emits toxic fumes of NOx and CN-. See also CALCIUM COMPOUNDS, AMIDES, and CYANIDE |
| Potential Exposure | Calcium cyanamide is used in agriculture as a fertilizer, herbicide; defoliant for cotton plants; and pesticide. It is also used in the manufacture of dicyandiamide and calcium cyanide as a desulfurizer in the iron and steel industry; and in steel hardening. |
| Carcinogenicity | Calcium cyanamide was weakly mutagenic in Salmonella typhimurium strain TA1535 and nonmutagenic in strain TA100. |
| Shipping | UN1403 Calcium cyanamide with .1% calcium carbide, Hazard Class: 4.3; Labels: 4.3-Dangerous when wet material |
| Incompatibilities | Commercial grades of calcium cyanamide may contain calcium carbide; contact with any form of moisture solutions may cause decomposition, liberating explosive acetylene gas and ammonia. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. May polymerize in water or alkaline solutions to dicyanamide. Contact with all solvents tested also causes decomposition |
Product picture

Packing &shipping&Payment
Shipping:by sea or by air
Payment:T/T,western union,moneygram
Packaging Details drum
Port:Tianjin
Lead Time :
| Quantity(Kilograms) | 1 - 10000 | >10000 |
| Est. Time(days) | 5 | To be negotiated |

 Company information
Company information
Hebei Mojin Biotechnology Co., Ltd, Our company is a professional in 4'-Methylacetophenone,Levamisole hydrochloride ,N-Methylformamide and other chemical reagents research and development production enterprises. Our business covers more than 30 countries, most of the big customers come from Europe, America and other countries in the world, we can guarantee the quality and price. In recent decades, with the efforts of all employees, we have established many cooperative companies in shandong, henan, guangdong and other places. Our corporate purpose is based on the market, enhance the strength, take the road of scientific and environmental sustainable development, relying on the country. Technology r & d center, increase the investment in r & d, based on the domestic market, expand the international market, manufacturing quality products, sincere service to the society, into a modern, ecological, scientific and technological enterprise world.
 Advantage
Advantage
In stock
Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $9.90/1KG |
VIP6Y
|
Hebei Guanlang Biotechnology Co., Ltd.
|
2021-11-08 | |
| $15.00/1KG |
Zhuozhou Wenxi import and Export Co., Ltd
|
2021-08-11 | ||
| $1.00/1KG |
VIP5Y
|
Career Henan Chemical Co
|
2019-12-23 | |
| $15.00/1KG |
Zhuozhou Wenxi import and Export Co., Ltd
|
2021-06-27 |
- Since: 2017-12-08
- Address: Building A, Enjoy city, Zhongshan East Road, Shijiazhuang city, Hebei province
13288715578
sales@hbmojin.com











 China
China