Ammonium formate: Applications and reactions
May 23,2023
General description
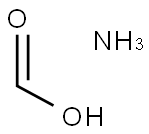
Ammonium formate is obtained by neutralizing formic acid and ammonia. Freeze formic acid and water, stir and pass ammonia gas to achieve a pH of 7-7.5, cool to -5 ℃, and filter to obtain ammonium formate. It is soluble in water and ethanol, and its aqueous solution is acidic. It is mainly used in the preparation of drugs and analytical reagents, as well as in electrolysis and capacitor industries. The preparation of ammonium formate was described in 1941. [1] It has been generally used in the precipitation of base metals from the salts of the noble metals. The use of ammonium formate in organic synthesis was first illustrated by Leucart, in which various carbonyl compounds were reacted with ammonium formate to afford the corresponding amines. This process was later named as the “Leucart Reaction”. The mechanism of Leucart reaction was studied by Wallach, and in 1949 a comprehensive review on the Leucart reaction was published by Moore. Later, the Leucart reaction was successful extended to the amination of 1,5-diketones and unsaturated ketones. Its appearance is as follows:

Figure 1 Appearance of Ammonium formate
Applications
Pure ammonium formate decomposes into formamide and water when heated, and this is its primary use in industry. Formic acid can also be obtained by reacting ammonium formate with a dilute acid, and since ammonium formate is also produced from formic acid, it can serve as a way of storing formic acid. Ammonium formate can also be used in palladium on carbon (Pd/C) reduction of functional groups. In the presence of Pd/C, ammonium formate decomposes to hydrogen, carbon dioxide, and ammonia. This hydrogen gas is adsorbed onto the surface of the palladium metal, where it can react with various functional groups. For example, alkenes can be reduced to alkanes, or formaldehyde to methanol. Activated single bonds to heteroatoms can also be replaced by hydrogens (hydrogenolysis). Ammonium formate can be used for reductive amination of aldehydes and ketones (Leuckart reaction). [2] Ammonium formate can be used as a buffer in high performance liquid chromatography (HPLC), and is suitable for use with liquid chromatography-mass spectrometry (LC/MS). The pKa values of formic acid and the ammonium ion are 3.8 and 9.2, respectively.
The ammonium formate palladium on carbon is very versatile, selective and rapid method for catalytic hydrogenolysis of wide variety of functionalities. Ammonium formate also has advantages of being readily available, inexpensive, stable and non-toxic and can be used in conjunction with either palladium on carbon (or other palladium derivates) or Raney-Nickel catalyst. Moreover it can be added to the reaction in a single portion and products can be easily separated from the reaction mixture.
Reactions
The use of ammonium formate as a reducing agent for functional groups in moderate reaction conditions is interesting and promising. Hydrazones and azines on reduction with formic acid or its derivatives such as ammonium formate gave good yields of corresponding hydrazines [3].
Insertion of amino group in the organic molecule via azide is a well know procedure. In past years a number of reagents have been developed for the reduction of azides to amino derivatives. Alkyl azides were successfully reduced to the corresponding primary amines in the presence of palladium/cabon, using ammonium formate as the hydrogen source. [4]
Furthermore, ammonium formate has been applied to the simultaneous deprotection and release of pentapeptide leucineenkephalin from the Merrifield peptide polystyrene resin under moderate reaction conditions and pressure in a neutral medium.
Ammonium formate with palladium carbon or palladium hydroxide on carbon/cyclohexene has been used for deprotection of O-benzyl group was selectively cleaved by catalytic hydrogenation using 10% palladium carbon and ammonium fomate as the hydrogen donor. [5]
References
[1]Johnstone, R.A.W., Wilby, A.H. Chem.Rev. 1985, 85, 129.
[2]Alexander, Elliot; Ruth Bowman Wildman (1948). "Studies on the Mechanism of the Leuckart Reaction". Journal of the American Chemical Society. 70: 1187–1189. doi:10.1021/ja01183a091.
[3]Kost, A.N., Grandberg, I.I. Zh. Obschch. Khim. 1955, 25, 1719; C.A. 1956, 50, 5544.
[4]Gartiser, T., Selve, C., Delpuech, J.J. Tetradedron Lett. 1983, 24, 1609.
[5]Hanessian, S., Liak, T.J., Vanasse, B. Synthesis 1981, 396.
- Related articles
- Related Qustion
- Applications of Ammonium Formate Nov 22, 2019
Ammonium formate, NH4HCO2, is the ammonium salt of formic acid. Ammonium formate is a colorless, hygroscopic, crystalline solid. Ammonium formate is a white solid with a weak odor of ammonia.
Astaxanthin is one of the most sought-after antioxidant supplements right now – and with good reason. It’s not only an antioxidant powerhouse but also jam-packed with anti-fatigue and anti-inflammator....
May 23,2023Food AdditivesAntioxidant 168 (tris(2,4-ditert-butylphenyl) phosphite) is a plastic additive mainly incorporated into Polypropylene (PP) and Polyethylene (PE) as an antioxidant.....
May 23,2023APIAmmonium formate
540-69-2You may like
Ammonium formate manufacturers
- Ammonium formate
-

- $1.00 / 1g
- 2024-04-30
- CAS:540-69-2
- Min. Order: 1g
- Purity: 99%
- Supply Ability: 1000kg
- Ammonium Formate
-

- $0.00 / 1Kg
- 2024-04-09
- CAS:540-69-2
- Min. Order: 1Kg
- Purity: 99.9%
- Supply Ability: 200tons
- Ammonium Formate
-

- $0.00 / 1kg
- 2024-04-05
- CAS:540-69-2
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 1000kg