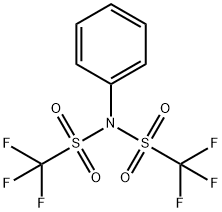
Application of N-Phenyl-bis(trifluoromethanesulfonimide) in synthesis reaction
Dec 10,2019
Description
N,N-Diethyl-p-phenylenediamine sulfate(PADMA) is used for the spectrophotometric determination of drugs such as salbutamol sulphate, ritodrine hydrochloride and isoxsuprine hydrochloride containing phenolic group. It is also used in the analysis of drugs like dapsone hydrochloride, sulfamethoxazole and sulfadiazine containing aromatic amino group. Further, it is used as a color developer and film developer in photography[1].
Application
Spectrophotometric methods are used for the determination of drugs containing a phenol group [salbutamol sulphate (SLB), ritodrine hydrochloride (RTD), isoxsuprine hydrochloride (IXP)] and drugs containing an aromatic amine group [dapsone hydrochloride (DAP), sulfamethoxazole (SFM), and sulfadiazine (SFD)] in pharmaceutical dosage forms.
Among the various methods available for the estimation of these drugs, such as HPLC, electrophoresis and gas chromatography, spectrophotometry is still the preferred technique due to its simplicity. Several spectrophotometric methods are available for the estimation of salbutamol, ritodrine, isoxsuprine, dapsone, sulfamethoxazole, and sulfadiazine. However, the mentioned methods have limitations such as the use of organic solvent extraction by evaporation, being time consuming and use of surfactants. Phenylenediamine and its derivatives have been widely used in the estimation of enzymes and drugs. So scientists developed a simple and sensitive method using N,N-diethyl-p-phenylenediamine sulphate (PADMA) and KIO4, to assay these drugs in bulk samples and in a wide variety of pharmaceutical preparations.
The proposed methods offer simple and accurate procedures, based on coupling of oxidized PADMA, for the determination of drugs containing phenolic and aromatic amine groups. As shown in the scheme 1, the coupling reaction takes place under slightly alkaline conditions for phenolic drugs because phenol is present as a more reactive phenoxide ion. Similarly, the reaction occurs in a slightly acidic environment for amine drugs because the concentration of quinine-diimine cation is maximal and at the same time an excessive amount of amine has not been converted to a nonreactive aminium salt (Scheme 1).
The optical parameters and statistical comparison justify the use of these methods in routine drug estimation in pure and dosage forms. Also, the comparison with the existing methods shows that the proposed procedures do not involve any critical reaction conditions such as pH control, use of organic solvents or tedious sample preparation steps and can be recommended for the routine analysis of drugs. After statistics validation, the method has high reliability [2].
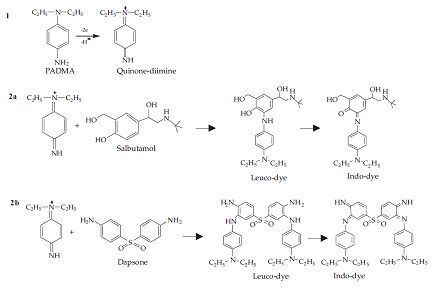
Scheme 1. Proposed reaction mechanism for the formation of an indo-dye
N,N-Diethyl-p-phenylenediamine sulfate salt has been used to prepare N,N-diethyl-p-phenylenediamine (DPD) solution, which is also commonly used as a chromogenic indicator for the determination of free and total chlorine by titrimetric, colorimetric and spectroscopic methods [3].
References
[1] https://www.alfa.com/en/catalog/A17832/
[2] Padmarajaiah Nagaraja, etc., Use of N,N-Diethyl-p-phenylenediamine Sulphate for the Spectrophotometric Determination of Some Phenolic and Amine Drugs
[3] https://www.sigmaaldrich.com/catalog/product/sial/07672?lang=en®ion=US
N-Phenyl-bis(trifluoromethanesulfonimide) could act as a transparent strong electron-withdrawing p-type dopant in carbon nanotubes, and it is the reactant for synthesis of amphoteric alpha-boryl aldehydes, enantioselective synthesis of core ring skeleton of leucosceptroids A-D and stereoselective sulfoxidation [1].
Application in synthesis reaction
N-Phenyl-bis(trifluoromethanesulfonimide)(PhNTF2) is stable, crystalline and easy-to-handle triflating agent, useful for the triflation of phenols and amines (especially aliphatic secondary amines; aromatic secondary amines). The reagent was introduced for the selective formation of trifluoromethanesulfonates (triflates) from phenols and amines, and it is often superior to trifluoromethanesulfonic anhydride for generating enol triflates from carbonyl compounds. Aryl and enol triflates are activated intermediates which can undergo Stille, Suzuki and other coupling reactions, and are frequently utilized in routes to sensitive, multifunctional molecules. Scheme 1 shows the example is found in the total synthesis of (±)-Gelsemine [2].
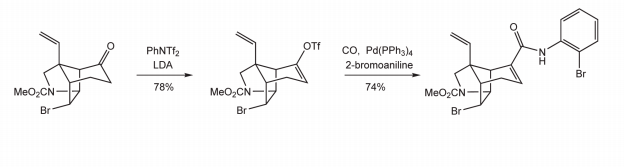
Scheme 1 The synthesis of reaction of (±)-Gelsemine
In another report, a conceptually new amination method of alcohols has been developed using α-hydroxy esters as substrates and N-phenyl bis-trifluoromethanesulfonimide (PhNTf2) as a test reagent [3]. α-Amino acids and their derivatives play a pivotal role in organic synthesis. The ease of preparation of α-hydroxy esters makes them ideal substrates for the preparation of α-amino esters. More recently, it has been demonstrated that a procedure based on the activation of a hydroxyl followed by displacement by a nucleophilic nitrogen source was more widely applicable. In this case, the triflate group has been recognized as the most convenient leaving group by comparison with usual bromide, mesylate, sulfonate, both in terms of efficiency and chirality.
The new amination reaction whereby the same reagent would serve a dual function of triflating and aminating an alcohol in a single operation. By capitalizing on the routine use of bis-trifluoromethanesulfonimides (triflimides) PhNTf2, N-pyridine bis-trifluoromethanesulfonimide (PyNTf2) and m-chloro-N-pyridine bis-trifluoromethanesulfonimide (m-Cl-PyNTf2) for trapping enolates to form vinyl triflates, in conjunction with the good nucleophilic properties of triflamides RNHTf and their anions, The first step of our planned reaction is proposed as follows: there is an initial addition of the alcoholate of the starting α-hydroxyester to a triflimide RNTf2, which may provide a transient triflate along with a nucleophilic residue -NRTf. Subsequently, the latter may easily substitute the triflate group to give the expected amino ester (Scheme 2).
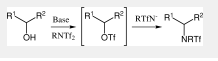
Scheme 2 Amination reaction with N-Phenyl-bis(trifluoromethanesulfonimide)
References
[1] https://www.sigmaaldrich.com/catalog/product/aldrich/78175?lang=en®ion=US
[2] A. Madin, C.J. O’Donnell, T. Oh, D.W. Old, L.E. Overman, M.J. Sharp, J. Am. Chem. Soc., 2005, 127, 18054.
[3] V. Gasparik, V. Dalla*, B. Decroix, A Conceptually New and Straightforward Method for the One-pot Transformation of Alcohols into Amines, Synlett 2002(3): 0528-0530.
- Related articles
- Related Qustion
- N-Phenyl-bis(trifluoromethanesulfonimide): Pharmaceutical Synthesis Applications and Green Preparation Apr 25, 2024
N-Phenyl-bis(trifluoromethanesulfonimide) aids in synthesizing antibacterial agents and LRRK2 kinase inhibitors and features an eco-friendly production method.
- N-Phenyl-bis(trifluoromethanesulfonimide): properties, applications and safety Jul 12, 2023
N-Phenyl-bis(trifluoromethanesulfonimide) is a stable, non-reactive solid used in organic synthesis and battery production with handling precautions.
- Application of N-Phenyl-bis(trifluoromethanesulfonimide) in synthesis reaction Dec 10, 2019
N-Phenyl-bis(trifluoromethanesulfonimide) could act as a transparent strong electron-withdrawing p-type dopant in carbon nanotubes, and it is the reactant for synthesis of amphoteric alpha-boryl aldehydes.
Polyethylene Glycol Monocetyl Ether is the tradename for polyethylene glycol hexadecyl ether , which is nonionic surfactant produced by the ethoxylation of cetyl alcohol to give a material with the general formula HO(C2H4O)nC16H33.....
Dec 10,2019SurfactantN-Phenyl-bis(trifluoromethanesulfonimide) could act as a transparent strong electron-withdrawing p-type dopant in carbon nanotubes, and it is the reactant for synthesis of amphoteric alpha-boryl aldehydes.....
Dec 10,2019Chemical ReagentsPolyethylene Glycol Monocetyl Ether is the tradename for polyethylene glycol hexadecyl ether , which is nonionic surfactant produced by the ethoxylation of cetyl alcohol to give a material with the general formula HO(C2H4O)nC16H33.....
Dec 10,2019SurfactantN-Phenyl-bis(trifluoromethanesulfonimide) could act as a transparent strong electron-withdrawing p-type dopant in carbon nanotubes, and it is the reactant for synthesis of amphoteric alpha-boryl aldehydes.....
Dec 10,2019Chemical ReagentsN-Phenyl-bis(trifluoromethanesulfonimide)
37595-74-7You may like
- Benzhydrol:Melting point,Uses,Hazards
Mar 22, 2024
- What is Methoxypolyethylene glycol amine used for?
Mar 14, 2024
- What is 1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine?
Mar 14, 2024
N-Phenyl-bis(trifluoromethanesulfonimide) manufacturers
- N,N-Bis(trifluoromethylsulfonyl)aniline
-

- $0.00 / 25KG
- 2024-04-30
- CAS:37595-74-7
- Min. Order: 1KG
- Purity: ≥99%
- Supply Ability: 200mt/year
- N-Phenyl-bis(trifluoromethanesulfonimide)
-

- $65.00 / 1kg
- 2024-01-02
- CAS:37595-74-7
- Min. Order: 10kg
- Purity: 0.99
- Supply Ability: 20tons
- N-Phenyl-bis(trifluoromethanesulfonimide)
-

- $0.00 / 25KG
- 2023-10-21
- CAS:37595-74-7
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 50000KG/month