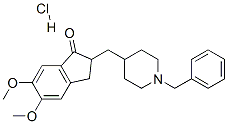
Donepezil hydrochloride: discovery, pharmacology and safety
Oct 16,2023
General Description
Donepezil hydrochloride was discovered through a challenging research journey starting in 1983. Initial studies focused on synthesizing tacrine derivatives to treat Alzheimer's Disease (AD). Although no nontoxic derivatives were found, a random screening process led to the identification of an N-benzylpiperazine derivative with antiAChE activity. Through a structure-activity relationship study, several key observations were made, leading to the discovery of compound 8, a benzamide derivative with high antiAChE activity. However, its poor bioavailability led to the design of an indanone derivative, which eventually led to the discovery of Donepezil as the most balanced compound. Donepezil hydrochloride, marketed as Aricept®, is now approved for treating mild to moderate AD. Pharmacological experiments demonstrated its inhibitory effects on acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE) activities. Donepezil hydrochloride exhibited favorable safety and tolerability profiles in Chinese clinical trials, with mild and transient side effects observed. It is highly likely to be efficacious for Chinese patients with AD.
Figure 1. Tablets of donepezil hydrochloride
Discovery
The discovery of Donepezil hydrochloride was a challenging and time-consuming journey that began in 1983 at the Tsukuba Research Laboratories of Eisai Co., Ltd. The initial research focused on synthesizing tacrine derivatives to find a potential treatment for Alzheimer's Disease (AD). Although no nontoxic tacrine derivatives were discovered, a random screening process led to the identification of an N-benzylpiperazine derivative (compound 1) with antiAChE activity. Further investigations revealed promising results when tested against electric eel AChE. However, the activity decreased when tested against rat brain AChE, leading to a year-long study on the structure-activity relationship (SAR) of phenyl ether derivatives. Through this study, several key observations were made, including the importance of bulky groups and the replacement of the piperazine moiety with piperidine. Based on these findings, the N-benzylpiperazine was replaced with N-benzylpiperidine, resulting in a significant increase in antiAChE activity. Additionally, the SAR study on benzamide derivatives yielded valuable insights, such as the role of substituents on the phenyl group and the nitrogen atom of the amide group. Following extensive synthesis and evaluation of compounds, compound 8, a benzamide derivative, exhibited the highest antiAChE activity. However, its poor bioavailability and short duration of action rendered it unsuitable for clinical testing. This setback prompted further screening, eventually leading to the design of an indanone derivative (16), which offered moderate AChE activity and a longer duration of action. Further synthesis and testing of indanone derivatives ultimately led to the discovery of Donepezil as the most balanced compound in terms of antiAChE activity. Donepezil hydrochloride has since been approved for the treatment of mild to moderate AD and is marketed under the trade name Aricept® in several countries. 1
Pharmacology
The initial experiments aimed to evaluate the inhibitory effects of donepezil hydrochloride on the activities of acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE). These effects were compared to two well-known cholinesterase inhibitors, physostigmine (PHY) and tacrine. Rat brain homogenates were used as the source of AChE, while rat plasma was utilized for BuChE. To assess the inhibitory potency, both enzyme preparations were incubated with various concentrations of each inhibitor. Acetylcholine (ACh) served as the substrate for AChE, while butyrylthiocholine (BuCh) acted as the substrate for BuChE. The inhibitory effects were measured, and the results were expressed as IC50 values, which indicate the concentration required to inhibit 50% of the enzyme activity. The obtained IC50 values are summarized in Table 1. These values provide a quantitative measure of the relative inhibitory effects of the different inhibitors on AChE and BuChE activities. By comparing these values, it is possible to determine the potency of donepezil hydrochloride compared to physostigmine and tacrine in inhibiting cholinesterase activity. In conclusion, these initial experiments aimed to assess the inhibitory effects of donepezil hydrochloride on AChE and BuChE activities, providing valuable insights into its potential as a cholinesterase inhibitor for therapeutic purposes. 2
Safety
Donepezil hydrochloride has been demonstrated to be safe and well tolerated in Chinese clinical trials, especially when compared to other medications such as placebo, huperzine A, galantamine, memantine, and tacrine. Dropout and adverse event rates during 12-24 weeks of treatment ranged from 0% to 8.4% and 4.8% to 47%, respectively, indicating a favorable safety profile. Chinese patients on lower dosages (5 mg/d) and shorter durations (12 or 16 weeks) of Donepezil hydrochloride treatment exhibited better safety and tolerability compared to patients in Western countries and Japan. Severe Chinese AD patients treated with a dosage of 10 mg/d also showed similar withdrawal rates and lower adverse event rates compared to Western studies. Common side effects of Donepezil hydrochloride in Chinese AD patients include dizziness, gastrointestinal symptoms, insomnia, fatigue, sinus bradycardia, Q-T interval prolongation, abnormal liver function tests, and agitation. However, most side effects were mild and transient, with spontaneous remission observed in long-term intervention. In comparison to galantamine, donepezil had a higher incidence of adverse events but had lower withdrawal rates. When used in combination with memantine, there were no significant differences in adverse event rates compared to other medications. Overall, Donepezil hydrochloride is highly likely to be efficacious for Chinese patients with mild to moderate AD and possibly effective for severe AD. It is generally safe and well tolerated, although caution should be exercised regarding gastrointestinal symptoms, bradycardia, and abnormal liver function. 3
Reference
1. Sugimoto H, Iimura Y, Yamanishi Y, Yamatsu K. Synthesis and structure-activity relationships of acetylcholinesterase inhibitors: 1-benzyl-4-[(5,6-dimethoxy-1-oxoindan-2-yl)methyl]piperidine hydrochloride and related compounds. J Med Chem. 1995 Nov 24;38(24):4821-4829.
2. Sugimoto H. Donepezil hydrochloride: a treatment drug for Alzheimer's disease. Chem Rec. 2001;1(1):63-73.
3. Zhang N, Gordon ML. Clinical efficacy and safety of donepezil in the treatment of Alzheimer's disease in Chinese patients. Clin Interv Aging. 2018 Oct 11;13:1963-1970.
- Related articles
- Related Qustion
- Donepezil Hydrochloride: Structure-Activity Relationships and Pharmacological Characteristics Mar 18, 2024
Donepezil hydrochloride is a potent inhibitor of acetylcholinesterase, showing enhanced potency through specific structural features and exhibiting significant neurochemical pharmacology.
- What is Donepezil Hydrochloride? Feb 26, 2020
Donepezil hydrochloride is a reversible inhibitor of the enzyme acetylcholinesterase, known chemically as (±)-2, 3-dihydro-5, 6-dimethoxy-2-[[1-(phenylmethyl)-4-piperidinyl]methyl]-1H-inden-1-one hydrochloride.
Dimethyl 1,3-acetonedicarboxylate is a highly reactive compound used in organic synthesis for diverse applications, such as UV protection and pharmaceutical development.....
Oct 16,2023APIp-Menthane-3,8-diol) is a natural insect repellent derived from lemon eucalyptus leaves, widely used to repel insects and protect against mosquito-borne diseases.....
Oct 16,2023APIDonepezil Hydrochloride
120011-70-3You may like
Donepezil Hydrochloride manufacturers
- Donepezil Hydrochloride
-

- $50.00 / 25kg
- 2024-04-23
- CAS:120011-70-3
- Min. Order: 1kg
- Purity: 99.9%
- Supply Ability: 200000kg
- Donepezil Hydrochloride
-

- $0.00 / 25Kg/Bag
- 2024-04-22
- CAS:120011-70-3
- Min. Order: 2Kg/Bag
- Purity: 99% up / GMP
- Supply Ability: 20 tons
- Donepezil HCl
-
- $0.00 / 25kg
- 2024-03-28
- CAS:120011-70-3
- Min. Order: 25kg
- Purity: 98%
- Supply Ability: Inquiry