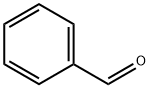
Introduction, Preparation, and Function of Benzaldehyde
Jul 20,2022
General description
Benzaldehyde is also known as benzoin aldehyde, benzaldehyde, phenylaldehyde, benzene carbonal. The molecular formula is C7H6O and the molecular weight is 106.12. Benzaldehyde is a compound generated by the direct connection of aldehyde group and phenyl group [1]. Because of its similar fragrance of bitter almond, benzaldehyde was once called bitter almond oil. Benzaldehyde is widely found in plant kingdom, especially in rosaceae, mainly in the form of glycosides, such as amygdalin in bitter almonds, in stem skins, leaves or seeds. Benzaldehyde naturally exists in bitter almond oil, patchouli oil, hyacinth oil, ylang ylang oil and other essential oils [2]. Benzaldehyde is chemically similar to aliphatic aldehydes, but there are differences. Benzaldehyde cannot be reduced by Fehring's reagent. In the reduction of benzaldehyde with aliphatic aldehydes, besides benzyl alcohol, some tetra-substituted o-diol compounds and homophenylglycol are also produced. In the presence of potassium cyanide, two molecules of benzaldehyde receive hydrogen atoms to form benzoin. Benzaldehyde can also carry out electrophilic substitution reactions on aromatic nuclei, mainly generating interposition substitution products. For example, the main product is m-nitrobenzaldehyde during nitrification [3].
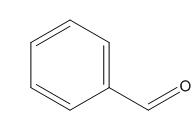
Fig. 1 The structure of benzaldehyde.
The physical and chemical indicators
The appearance of benzaldehyde shows colorless liquid. Its aroma is similar to bitter almond. The melting point is -26℃, the boiling point is 178℃[4], the relative density is 1.0415 (10/4℃), the refractive index is 1.544-1.547 [5], and the olubility is miscible with ethanol, ether, chloroform and so on,but slightly soluble in water.
Preparation
In industry, benzaldehyde is mainly oxidized by toluene through air or oxygen under the action of catalyst (vanadium pentoxide, tungsten trioxide or molybdenum trioxide). Or toluene is chlorinated into benzyl chloride under light, and then hydrolyzed and oxidized to prepare benzaldehyde [6]. It can also be chlorinated into dichloromethyl benzene and then hydrolyzed. Moreover, benzene is also used as raw material to react with carbon monoxide and hydrogen chloride under pressure and under the action of aluminum trichloride. In the laboratory, benzaldehyde is prepared by catalytic reduction of benzoyl chloride.
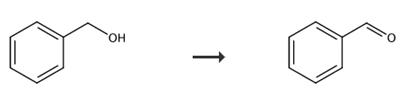
Fig. 2 The synthetic route of benzaldehyde [7].
(1) Oxidation or dehydrogenation of alcohols to aldehydes and ketones.

Fig. 3 The synthetic route of benzaldehyde [8].
(2) R: K3PO4, C:Co (N doped carbon-supported/N doped carbon-di), S:t-BuOH, 24 h, 150°C. ealed tube used, reusable catalyst, nitrogen-doped carbon supported coblat nanoparticle catalyst prepared and used, green chemistry, regioselective, solid-supported catalyst, thermal, Reactants: 2, Reagents: 1, Catalysts: 1, Solvents: 1, Steps: 1, Stages: 1, Most stages in any one step: 1.

Fig. 4 The synthetic route of benzaldehyde [9].
(3) R:O2, S:CH2Cl2, 12 h, 120°C, 0.1 MPa. Optimization study, optimized on catalyst, temperature,time and pressure, FeSBA-15 catalyst used, low pressure, thermal, Reactants: 1, Reagents: 1, Solvents: 1, Steps: 1, Stages: 1, Most stages in any one step: 1.
Safety assessment of benzaldehyde
Benzaldehyde is an aromatic aldehyde used in cosmetics as a denaturant, a flavoring agent, and as a fragrance. Currently used in only seven cosmetic products, its highest reported concentration of use was 0.5% in perfumes. Benzaldehyde is a generally regarded as safe (GRAS) food additive in the United States and is accepted as a flavoring substance in the European Union. Because Benzaldehyde rapidly metabolizes to Benzoic Acid in the skin, the available dermal irritation and sensitization data demonstrating no adverse reactions to Benzoic Acid were considered supportive of the safety of Benzaldehyde. Benzaldehyde is absorbed through skin and by the lungs, distributes to all well-perfused organs, but does not accumulate in any specific tissue type. After being metabolized to benzoic acid, conjugates are formed with glycine or glucuronic acid, and excreted in the urine [10, 11]. Little acute toxicity was seen. The oral LD(50) of Benzaldehyde in rats and mice ranged from 800 to 2850 mg/kg. The intraperitoneal LD(50) in white rats was 3265 mg/kg. In short-term oral studies, the no observed adverse effect level (NOAEL) was 400 mg/kg in rats and mice. In subchronic oral studies, the NOAEL was 400 mg/kg in rats and 600 mg/kg in mice. In a 16-week feeding study, rats given up to 10,000 ppm showed no signs of toxicity. Repeated inhalation of volatilized Benzaldehyde produced ocular and nasal irritation at 500 ppm and death in rabbits at 750 ppm [12]. Although there are limited irritation and sensitization data available for Benzaldehyde, the available dermal irritation and sensitization data and ultraviolet (UV) absorption and phototoxicity data demonstrating no adverse reactions to Benzoic Acid support the safety of Benzaldehyde as currently used in cosmetic products.
Application
Benzaldehyde is an important raw material in the pharmaceutical, dye, perfume and resin industries. It is mainly used in the manufacture of lauraldehyde, lauric acid, magenta, etc. it can also be used as a solvent, plasticizer and low-temperature lubricant [13]. In the essence industry, it is mainly used to prepare edible essence, and a small amount is used in daily chemical essence and cigarette essence.
Hazard Overview
Health hazard: benzaldehyde can stimulate the mucous membrane of eyes and respiratory tract. Due to its low volatility, its stimulating effect is insufficient to cause serious harm [14]. Benzaldehyde is flammable, toxic and irritating.
First aid measures
As for skin contact, if touching benzaldehyde, please take off contaminated clothing and rinse with running water. When your eyes contact benzaldehyde, you should lift eyelid, rinse with running water or normal saline. If necessary, go to a doctor. If breathing is difficult, give oxygen. When eating benzaldehyde, you should drink enough warm water to induce vomiting. Go to a doctor.
Fire control measures
Benzaldehyde is flammable in case of open fire and high heat. At high heat, the pressure inside the vessel increases, causing the danger of cracking and explosion [15]. Harmful combustion products of benzaldehyde include carbon monoxide, carbon dioxide. If benzaldehyde causes fire, fire fighters should wear gas masks and full-body fire fighting clothes to fight fire upwind. If possible, move containers from the fire to an open area. Spray water to keep the fire container cool until the end of the fire. Containers in the fire must be evacuated immediately if they become discolored or produce sound from the safety relief device [16].
Operation, disposal, storage and transportation
Precautions for operation of benzaldehyde comprises the following steps: closed operation, full exhaust. Operators must be specially trained and strictly abide by the operating procedures. It is recommended that the operator wear a self-priming filter gas mask (half mask), chemical safety glasses, protective clothing and rubber oil-resistant gloves. Keep away from fire and heat source. No smoking in workplace. Use explosion-proof ventilation systems and equipment. Prevent steam leakage into the workplace air. Avoid contact with oxidants and acids [17]. Operate and dispose in nitrogen. Handling should be carried lightly to prevent damage to packaging and containers. Equipped with the corresponding variety and quantity of firefighting equipment and leakage emergency treatment equipment. Empty containers may contain hazardous materials.
Storage precautions of benzaldehyde are described as follows: Store in a cool and ventilated warehouse. Keep away from fire and heat source. Packing should be sealed and out of contact with air. It should be stored separately from oxidants, acids and edible chemicals, and must not be mixed. Explosion-proof lighting and ventilation facilities are adopted [18]. Do not use mechanical equipment and tools that may cause sparks. The storage area shall be equipped with leakage emergency treatment equipment and suitable storage materials.
Transportation Precautions of benzaldehyde are illustrated: before transportation, check whether the packaging container is complete and sealed, and ensure that the container does not leak, collapse, fall, and damage during transportation. It is forbidden to mix with oxidizer, acid and edible chemicals [19]. Transport vehicles and vessels must be thoroughly cleaned and disinfected, otherwise other articles may not be carried. When shipping, the installation position should be far away from the bedroom, kitchen, and isolated from the engine room, power supply, fire source and other parts.
References
[1] A. Andersen, Final report on the safety assessment of benzaldehyde, International journal of toxicology 25 (2006) 11-27.
[2] M.A. Andrade, L.M. Martins, Selective styrene oxidation to benzaldehyde over recently developed heterogeneous catalysts, Molecules 26(6) (2021) 1680.
[3] L. Bao, P. Lv, T. Fei, Y. Liu, C. Sun, S. Pang, Crystal structure and explosive performance of a new CL-20/benzaldehyde cocrystal, Journal of Molecular Structure 1215 (2020) 128267.
[4] M. Berger, I.L. Goldblatt, C. Steel, Photochemistry of benzaldehyde, Journal of the American Chemical Society 95(6) (1973) 1717-1725.
[5] A. Corma, V. Fornes, R. Martin-Aranda, F. Rey, Determination of base properties of hydrotalcites: condensation of benzaldehyde with ethyl acetoacetate, Journal of Catalysis 134(1) (1992) 58-65.
[6] D. Dolphin, Porphyrinogens and porphodimethenes, intermediates in the synthesis of meso‐tetraphenylporphins from pyrroles and benzaldehyde, Journal of Heterocyclic Chemistry 7(2) (1970) 275-283.
[7] B. Goldfuss, M. Steigelmann, S.I. Khan, K. Houk, Rationalization of enantioselectivities in dialkylzinc additions to benzaldehyde catalyzed by fenchone derivatives, The Journal of Organic Chemistry 65(1) (2000) 77-82.
[8] D. Haffad, U. Kameswari, M. Bettahar, A. Chambellan, J. Lavalley, Reduction of benzaldehyde on metal oxides, Journal of Catalysis 172(1) (1997) 85-92.
[9] R.K. Kakar, E.A. Rinehart, C. Quade, T. Kojima, Microwave spectrum of benzaldehyde, The Journal of Chemical Physics 52(7) (1970) 3803-3813.
[10] M. Kochi, S. Takeuchi, T. Mizutani, K. Mochizuki, Y. Matsumoto, Y. Saito, Antitumor activity of benzaldehyde, Cancer Treat Rep 64(1) (1980) 21-23.
[11] L. Kosmider, A. Sobczak, A. Prokopowicz, J. Kurek, M. Zaciera, J. Knysak, D. Smith, M.L. Goniewicz, Cherry-flavoured electronic cigarettes expose users to the inhalation irritant, benzaldehyde, Thorax 71(4) (2016) 376-377.
[12] H. Lampert, W. Mikenda, A. Karpfen, Molecular geometries and vibrational spectra of phenol, benzaldehyde, and salicylaldehyde: experimental versus quantum chemical data, The Journal of Physical Chemistry A 101(12) (1997) 2254-2263.
[13] M.N. Nierop Groot, J.A. de Bont, Conversion of phenylalanine to benzaldehyde initiated by an aminotransferase in Lactobacillus plantarum, Applied and Environmental Microbiology 64(8) (1998) 3009-3013.
[14] A. Padwa, W. Bergmark, D. Pashayan, Photoreduction of benzaldehyde N-alkylimines, Journal of the American Chemical Society 90(16) (1968) 4458-4459.
[15] M. Ponzi, C. Duschatzky, A. Carrascull, E. Ponzi, Obtaining benzaldehyde via promoted V2O5 catalysts, Applied Catalysis A: General 169(2) (1998) 373-379.
[16] M. Reetz, M. Huellmann, W. Massa, S. Berger, P. Rademacher, P. Heymanns, Structure and electronic nature of the benzaldehyde/boron trifluoride adduct, Journal of the American Chemical Society 108(9) (1986) 2405-2408.
[17] S. Saito, T. Ohwada, K. Shudo, Friedel-Crafts-type reaction of benzaldehyde with benzene. Diprotonated benzaldehyde as the reactive intermediate, Journal of the American Chemical Society 117(45) (1995) 11081-11084.
[18] G.W. Stacy, B.V. Ettling, A.J. Papa, Reactions of Benzaldehyde with o-Nitroaniline1, The Journal of Organic Chemistry 29(6) (1964) 1537-1540.
[19] S. Takeuchi, M. Kochi, K. Sakaguchi, K. Nakagawa, T. Mizutani, Benzaldehyde as a carcinostatic principle in figs, Agricultural and Biological Chemistry 42(7) (1978) 1449-1451.
- Related articles
- Related Qustion
- Polarity of Benzaldehyde Dec 27, 2023
Benzaldehyde (C6H5CHO) is an organic compound consisting of a benzene ring with a formyl substituent. It is the simplest aromatic aldehyde and one of the most industrially useful.
- Structure, Intermolecular Interactions and Applications of Enzaldehyde Dec 27, 2023
Benzaldehyde represents the simplest aromatic aldehyde and the most used industrially. This article will introduce its structure, intermolecular interactions and applications.
Ursolic acid is a pentacyclic triterpene acid present in many plants, including apples, bilberries, cranberries, elder flower, peppermint, lavender, oregano, thyme, hawthorn, prunes.....
Jul 20,2022Biochemical EngineeringTianeptine sodium salt not only has good antidepressant effect, but also has fewer adverse reactions than traditional tricyclic antidepressants, almost no adverse effects on cardiovascular system.....
Jul 20,2022APIBenzaldehyde
100-52-7You may like
- Benzaldehyde
-

- $10.00 / 1kg
- 2024-04-29
- CAS:100-52-7
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 300tons
- Benzaldehyde
-

- $15.00/ kg
- 2024-04-23
- CAS:100-52-7
- Min. Order: 1kg
- Purity: 99.912%
- Supply Ability: 10ton
- Flavor Intermediate Benzaldehyde
-

- $0.00 / 1kg
- 2024-04-22
- CAS:100-52-7
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 100 tons