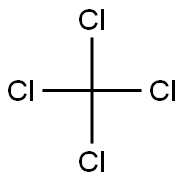
Polarity of Carbon tetrachloride(CCl4)
Dec 20,2023
Polarity of Carbon tetrachloride(CCl4)
CCl4 is an organic compound that can be formed by the halogenation of methane and chlorine in the presence of sunlight. Chlorine is arranged tetrahedrally around the carbon atoms, resulting in a symmetrical arrangement. The polarity of the bond is a result of the separation of some of the charges in the covalent bond due to differences in electronegativity.
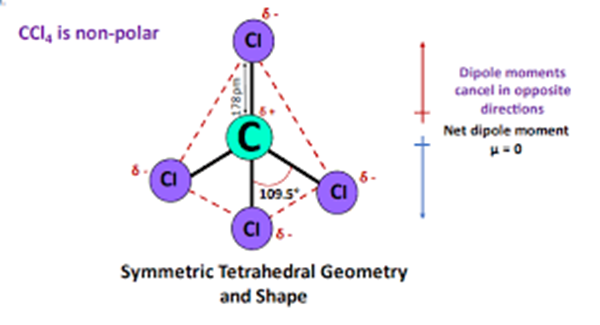
Chlorine is a halogen with a high effective nuclear charge, which leads to a high electronegativity, giving the carbon atom a slight positive charge. This difference in electronegativity between carbon and chlorine makes their bond polar. However, bond polarity by itself does not determine the properties of the entire compound. Since the four equidistant bonds are symmetrically arranged, the dipole moment in them points towards the more electronegative chlorine atom. When the magnitudes are the same and the directions are opposite, the dipole moments as vectors are cancelled out. The dipole moment of one bond cancels out the dipole moment of the other bond that is opposite to it. Thus, the two pairs of bonds in carbon tetrachloride cancel each other out, resulting in a net zero dipole moment.
Therefore carbon tetrachloride CCl4 is a nonpolar molecule.
- Related articles
- Related Qustion
- Use of Carbon tetrachloride Dec 20, 2021
Carbon tetrachloride is a manufactured chemical and does not occur naturally in the environment. It is produced by chlorination of a variety of low molecular weight hydrocarbons such as carbon disulfide, methane, ethane, propane, or ethylen
- Carbon tetrachloride -Health Hazard and Toxicity Sep 11, 2019
Carbon tetrachloride, also known as tetrachloromethane, has its molecule formula being CCl4. It appears as colorless liquid with the melting point of-23 ° C, boiling point of 76.8 ° C and the relative density of 1.5867. It can dissolve grea
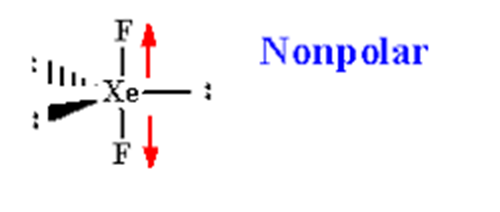
XeF2 is a non-polar molecule because the fluorine molecules on either side of the central atom do not have dipole moments and therefore have no polarity.....
Dec 20,2023API8-hydroxyquinoline can be used as a raw material for the synthesis of corrosion inhibitors. And studies have reported the corrosion inhibition of 8-hydroxyquinoline derivatives on N80 steel in 1 M hydrochloric acid medium.....
Dec 20,2023API