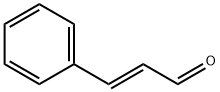
Trans-cinnamaldehyde: Pharmacodynamics, Mechanism of action, Toxicity, Application, Preparation
Apr 3,2023
Trans-cinnamaldehyde[1] is a natural compound found in cinnamon essential oil. It is an organic compound with the chemical formula C9H8O, and it has a characteristic sweet and spicy odor. Trans-cinnamaldehyde has been widely studied for its pharmacological properties, which include antimicrobial, anti-inflammatory, and antioxidant activities. These properties make it a promising candidate for the development of new drugs and therapies.
Trans-cinnamaldehyde has also shown potential as a cytotoxic agent against cancer cells. Its ability to induce apoptosis in various cancer cell lines has been demonstrated in numerous studies. Additionally, trans-cinnamaldehyde has been found to regulate glucose metabolism, possibly through the activation of insulin receptors and the inhibition of glucose uptake by the liver.
Trans-cinnamaldehyde is a versatile and promising compound with diverse pharmacological properties. Its potential therapeutic applications range from the treatment of microbial infections and inflammation-related disorders to metabolic diseases and cancer. Further research on this compound may lead to the development of novel therapeutics that could improve health outcomes for many people.
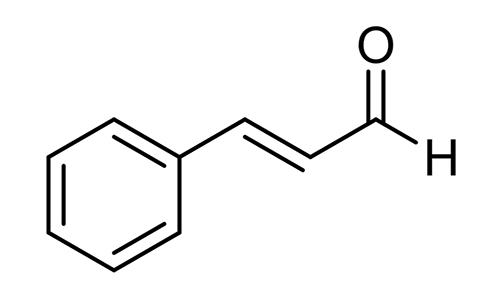
Figure 1 the molecular formula of Trans-cinnamaldehyde
Pharmacodynamics
Pharmacodynamics of trans-cinnamaldehyde mainly involve its ability to interact with various cellular targets, leading to changes in cellular signaling pathways and physiological responses[2].
One of the main pharmacological actions of trans-cinnamaldehyde is its antimicrobial activity. It has been shown to inhibit the growth of a wide range of bacteria, fungi, and viruses, possibly by disrupting their cell membranes or inhibiting enzyme activity. Additionally, trans-cinnamaldehyde has been found to have anti-inflammatory effects, which may be attributed to its ability to inhibit the production of inflammatory cytokines and chemokines.
Other pharmacodynamic effects of trans-cinnamaldehyde include its ability to regulate glucose metabolism in the body, potentially through the activation of insulin receptors and the inhibition of glucose uptake by the liver. It has also been found to possess antioxidant properties, which could help protect against oxidative stress and associated diseases.
Overall, the pharmacodynamics of trans-cinnamaldehyde are diverse and involve multiple biochemical pathways. Its potential therapeutic applications include the treatment of microbial infections, inflammation-related disorders, metabolic diseases, and oxidative stress-related conditions.
Mechanism of action
The mechanism of action of trans cinnamaldehyde involves its ability to interact with various cellular targets, leading to changes in cellular signaling pathways and physiological responses.
One of the main mechanisms of action is its ability to disrupt the cell membrane of microorganisms, leading to their death. Trans-cinnamaldehyde can penetrate the cell membrane of bacteria, fungi, and viruses, disrupting their cellular structure and function, ultimately resulting in their death.
Another mechanism of action of trans-cinnamaldehyde is its ability to inhibit the production of inflammatory cytokines and chemokines, which are involved in the development of inflammation. This inhibition occurs through the modulation of intracellular signaling pathways, such as NF-κB and MAPKs, which regulate the expression of genes involved in inflammation.
Trans-cinnamaldehyde also acts as an insulin sensitizer, by stimulating glucose uptake and metabolism in skeletal muscle cells, and by inhibiting glucose production in the liver. This effect occurs through the activation of the AMP-activated protein kinase (AMPK) pathway, which plays a critical role in regulating glucose homeostasis.
Furthermore, trans-cinnamaldehyde has been found to possess antioxidant properties, which could help protect against oxidative stress and associated diseases. Its antioxidant activity is attributed to its ability to scavenge free radicals and prevent lipid peroxidation.
The mechanism of action of trans-cinnamaldehyde is diverse and involves multiple biochemical pathways. Its unique molecular structure allows it to interact with various cellular targets, making it a promising candidate for the development of new drugs and therapies.
Toxicity
The trans-cinnamaldehyde can be toxic if consumed or used improperly. Ingesting large amounts of cinnamon or cinnamon-containing supplements can cause liver damage or other adverse health effects. In addition, exposure to high levels of trans-cinnamaldehyde can irritate the skin and eyes and may cause respiratory problems in sensitive individuals. It is important to follow proper safety precautions when handling or using this compound, and to consult with a healthcare professional before consuming or using cinnamon supplements or products containing this ingredient[3].
Application
The inhibitory mechanism of trans-cinnamaldehyde on Vibrio parahaemolyticus and its control effect on Vibrio parahaemolyticus in fresh shrimp. The minimum inhibitory mass concentration of trans-cinnamaldehyde to 6 strains of Vibrio parahaemolyticus was 50-70ug/mL, which significantly reduced the maximum growth rate and maximum optical density of the bacteria. Moreover, trans-cinnamaldehyde significantly reduces the integrity of the cell membrane of Vibrio parahaemolyticus and changes the shape of the bacteria, causing the bacteria to shrivel and collapse. Trans-cinnamaldehyde has a good antibacterial effect on Vibrio parahaemolyticus in fresh shrimp , and the antibacterial effect increases with the increase of its mass concentration. When the mass concentration of trans-cinnamaldehyde is 0.4 mg/ mL, when the mass concentration of trans-cinnamaldehyde acts on fresh shrimp at 4 °C, all Vibrio parahaemolyticus in fresh shrimp is reduced to detectable in 1 h. In summary, trans-cinnamaldehyde has a good antibacterial effect on Vibrio parahaemolyticus, and it exerts its antibacterial effect by reducing the integrity of the cell membrane of Vibrio parahaemolyticus, making the cells shriveled and shrunken. Functional. Combined with its antibacterial effect on Vibrio parahaemolyticus in fresh shrimp, trans-cinnamaldehyde has the potential to be used as a natural antibacterial agent to control the infection of Vibrio parahaemolyticus in fresh shrimp and other seafood[4].
The minimum inhibitory concentration of Trans nmamadelyde vs C Sakazakii is 0.4 mg/ mL and can effectively prolong the growth retardation period of C Sakazakii; Trans nmamadelyde at concentrations of MIC and 2 MIC reduced the membrane integrity of C sakazakii cells to 60 % and 46%, and caused its cell membrane to shrink and the cell body to shrivel; Trans nmamadelyde at a concentration of 0.4 % (V/V) can reduce the amount of C sakazakii in reconstituted infant formula milk to below the detection limit in 90 min (25 ℃) and 60 min (37 ℃). Trans nmamadelyde has the potential to be used as a natural antibacterial substance in foods such as milk powder, thereby effectively preventing and reducing foodborne diseases caused by Cronobacter sakazakii[5].
The antibacterial mechanism of trans cinnamaldehyde against Sclerotinia oryzae and its effect on the activities of nicotinamide adenine dinucleoside malate dehydrogenase (NADMDase), succinate dehydrogenase (SDHase), and adenosine triphosphate dehydrogenase (ATPase) have been studied. The result shows that after 4 μg/mL of trans cinnamaldehyde’s treatment, the number of liposomes in the mycelial cells increased significantly, and the mitochondrial structure became blurred. When the mass concentration of trans cinnamaldehyde increases to 30 μg/mL, it can destroy the integrity of the mycelium cell wall, and has a relative inhibition rate of 44.3% on NAD-MDHase activity and 76.7% on SDHase activity. The activities of Na+- K+- ATPase, Ca2+- Mg2+- ATPase, Ca2+- ATPase, and Mg2+- ATPase change in a U-shape with the increase in the mass concentration of trans cinnamaldehyde, with 4 μ G/mL of trans cinnamaldehyde has the strongest inhibitory effect on ATPase activity, with a relative inhibitory rate of over 78.4%. Cell walls and mitochondria may be the targets of trans cinnamaldehyde in inhibiting the mycelial growth of Rhizoctonia oryzae[6].
Preparation
Trans-cinnamaldehyde is an organic compound with the chemical formula C9H8O. It is a pale yellow liquid with a sweet, spicy odor that is commonly used as a flavoring agent in food, beverages, and personal care products.
The traditional method for preparing trans-cinnamaldehyde involves the oxidation of cinnamyl alcohol using potassium permanganate. Here are the steps:
1. Dissolve 10 g of cinnamyl alcohol in 50 mL of glacial acetic acid.
2. Slowly add 6 g of powdered potassium permanganate to the solution while stirring constantly. The reaction mixture will turn dark purple.
3. Heat the mixture to 60-70°C and continue stirring for 30 minutes.
4. Allow the mixture to cool, then filter off the dark brown precipitate using a Buchner funnel.
5. To the filtrate, slowly add sodium bisulfite solution until the color changes from purple to pale yellow. This serves to remove excess permanganate.
6. Extract the yellow oil with dichloromethane or diethyl ether.
7. Dry the extract with anhydrous magnesium sulfate, then evaporate the solvent under reduced pressure to obtain trans-cinnamaldehyde as a pale yellow liquid.
Note that this is just one method for preparing trans-cinnamaldehyde, and there may be other methods that are more suitable depending on the specific application. Additionally, handling potassium permanganate can be dangerous and should only be done by trained professionals with proper safety equipment.
References
[1] Belli Aaron J, Molta Gavin J, Gordon Patrick M, et al. Further studies on green crossed aldol condensation reactions of trans-cinnamaldehyde and derivatives[J]. Abstracts of Papers of The American Chemical Society,2010,240.
[2] Wang Shu, Kang OkHwa, Kwon DongYeul. Trans-Cinnamaldehyde Exhibits Synergy with Conventional Antibiotic against Methicillin-Resistant Staphylococcus aureus[J]. International Journal of Molecular Sciences,2021,22(5).
[3] Ranjitkar Saurav, Zhang Delong, Sun Fei, et al. Cytotoxic effects on cancerous and non-cancerous cells of trans-cinnamaldehyde, carvacrol, and eugenol.[J]. Scientific reports,2021,11(1).
[4] Guo Du, Zhang Wenting, Hou Xusheng, et al. Inhibitory effect of trans-cinnamon on parahaemolytic bacteria [J]. Journal of Food and Biotechnology, 2020,39(01):14-23.
[5] Shi Chao, Guo Du, Zhang Wenting, et al. Study on the inhibitory effect of trans cinnamaldehyde on Cronobacter sakazakii [J]. Modern Food Science and Technology, 2017,33 (10): 58-66.
[6] Zheng Jingge, Guo Zhixin, Liu Tingting, et al. The effect of trans cinnamaldehyde on the cell ultrastructure and the activity of three respiratory enzymes of Rhizoctonia oryzae [J]. Journal of Pesticide Science, 2019,21 (02): 158-164.
- Related articles
- Related Qustion
- trans-Cinnamaldehyde: A Natural Compound with Potentiating Effects in Bacteria and Fungi Feb 5, 2024
trans-Cinnamaldehyde, derived from cinnamon bark, shows potential in combating antibiotic-resistant bacteria and enhancing antifungal treatments.
Canagliflozin hemihydrate (Invokana) is a C-glucoside with a thiophene ring that is an orally available inhibitor of sodium-glucose transporter 2 (SGLT2) with antihyperglycemic activity.....
Apr 3,2023DrugsPotassium carbonate is the primary component of potash and the more refined pearl ash or salts of tartar. It is prepared commercially by the reaction potassium hydroxide with carbon dioxide.....
Apr 3,2023Inorganic saltstrans-Cinnamaldehyde
14371-10-9You may like
- Geranyl acetone: Properties, Production process and Uses
Mar 6, 2024
- What is terpineol used for?
Feb 26, 2024
- Structure and Polarity of 1,4-Dimethoxybenzene
Jan 3, 2024
trans-Cinnamaldehyde manufacturers
- trans-Cinnamaldehyde
-

- $5.00 / 200kg
- 2024-04-29
- CAS:14371-10-9
- Min. Order: 1kg
- Purity: ≥99%
- Supply Ability: 200mt/year
- trans-Cinnamaldehyde
-

- $20.00 / 25kg
- 2024-04-27
- CAS:14371-10-9
- Min. Order: 1kg
- Purity: 99.9%
- Supply Ability: 200000kg
- Trans-Cinnamaldehyde
-

- $0.00 / 1Kg
- 2024-04-07
- CAS:14371-10-9
- Min. Order: 1Kg
- Purity: 99.9%
- Supply Ability: 200 tons