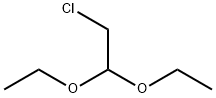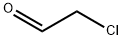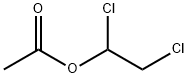
1,2-Dichloroethyl acetate synthesis
- Product Name:1,2-Dichloroethyl acetate
- CAS Number:10140-87-1
- Molecular formula:C4H6Cl2O2
- Molecular Weight:157
Yield:621-62-5 790 grams (56 % pure)
Steps:
1 EXAMPLE 1
EXAMPLE 1 Procedure A - To a 1-liter jacketed resin kettle equipped with a mechanical stirrer, gas dispersion tube and thermometer, and cooled to -5° C. with circulating trichloroethylene thermostated cooling liquid, was charged 250 grams (2.9 moles) of vinyl acetate (inhibited with 300 parts per million diphenylamine). Chlorine gas (230.0 grams, 3.2 moles; calculated 207.0 grams, 2.9 moles) was bubbled into the stirred solution from a preweighed lecture bottle over a 4.5-hour period at a rate to maintain the reaction mixture's temperature at about 15° C. (approximately 500 milliliters per minute flow rate). A precipitate initially formed in the reaction mixture but dissolved after about 1 hour. After the 4.50-hour period, the vinyl acetate was completely consumed, as determined via nuclear magnetic resonance spectroscopy. Nuclear magnetic resonance spectroscopic analysis of the product mixture showed the presence of 1,2-dichloroethyl acetate, chloroacetaldehyde, acetyl chloride, and unidentified derivatives of chloroacetaldehyde. The crude 1,2-dichloroethyl acetate thus obtained was transferred to a 5-liter flask equipped with a mechanical stirrer and thermometer, and cooled in an ice bath. Absolute ethanol (401.0 grams, 8.7 moles) was added to the stirred, cooled mixture over a 40-minute period at a rate sufficient to keep the temperature below 25° C. The first 30 percent of the addition was slow as the reaction is initially exothermic; the remaining 70 percent was added more quickly. The solution was allowed to stand, with stirring, at ambient temperatures overnight (about 16 hours), and thereafter heated under reflux for a total of 3.5 hours. The reaction mixture was cooled to ambient temperatures to yield 790 grams (56 percent pure) of crude chloroacetal solution. The hydrogen chloride gas from the refluxing reaction mixture was trapped in water and titrated to the neutral point with 80.0 grams (2.0 moles) of solid sodium hydroxide. Therefore, of the original 881.0 grams charged, 863.0 grams or 98 percent was recovered.
References:
US4189610,1980,A
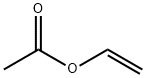
108-05-4
562 suppliers
$4.00/1000g

10140-87-1
28 suppliers
inquiry