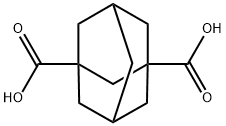
1,3-Adamantanedicarboxylic acid synthesis
- Product Name:1,3-Adamantanedicarboxylic acid
- CAS Number:39269-10-8
- Molecular formula:C12H16O4
- Molecular Weight:224.25
Efficient Synthesis of 1,3-Adamantanedicarboxylic Acid and 1,3-Diaminoadamantane
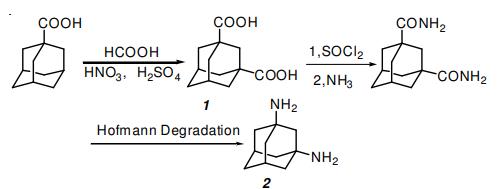
Reaction: 1-Adamantane carboxylic acid (20 g), nitric acid (65 %, 20 mL) and sulfuric acid (98 %, 160 mL) were placed in a three-necked roundbottom flask fitted with an efficient magnetic stirrer and a thermometer. Cooled to 0 oC and held at 0 oC, anhydrous formic acid (80 %, 70 mL) was added dropwise in 5 h and reacted for 1 h. poured to crushed ice, filtered and then washed the precipitate several times with water to give a white solid. Then the white solid was dissolved in aqueous NaOH solution and the upper clear solution was separated and acidified by HCl to pH = 3. Filtered, washed with water, dried in vacuum, recrystallized from ethanol. White solid, yield 22.9 g (92 %). m.p.: 275- 276 oC
1H NMR (DMSO-d6, 400 MHz) δ: 1.616 (m, 2H), 1.691-1.727 (m, 4H), 1.759-1.791 (m, 4H), 1.850-1.882 (m, 2H), 2.059 (m, 1H), 12.062 (br, s, 2H, COOH); 13C NMR (100 MHz, DMSO-d6) δ 27.37 (C-5, C-7), 34.98 (C-6), 37.66 (C-4, C-8, C-9, C-10), 39.78 (C-2), 39.89 (C-2, C-3), 177.76 (COOH). IR (KBr, νmax, cm-1) : 2913, 2851, 2636, 1691, 1451, 1410, 1344, 1249, 1103, 1084, 952, 743, 670, 528; Anal. calcd for C12H16O4: C 64.29, H 7.14; found C 64.55, H 7.22.

64-18-6
1056 suppliers
$22.12/250ML
5001-18-3
311 suppliers
$6.00/1g

39269-10-8
180 suppliers
$9.00/1g
Yield:39269-10-8 85%
Reaction Conditions:
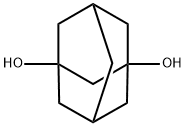
Stage #1: 1,3-adamantandiolwith sulfuric acid in water at 20; for 1 h;
Stage #2: formic acid in water at 0 - 20; for 4 h;
Stage #3: with sodium hydroxide;sulfuric acidmore than 3 stages;
Steps:
1.1 Example 1; [Synthesis of 1,3-adamantanedicarboxylic acid-bis(2'-methyl-2'-adamantyl) ester]; (1) Synthesis of 1,3-adamantanedicarboxylic acid
In a one liter three-neck flask equipped with a dropping funnel were placed 21.515 g (127.9 millimol) of 1,3-adamantanediol and 200 milliliter (mL) of 95% by mass of sulfuric acid, so that a uniform solution was obtained over a period of one hour at room temperature. Subsequently, the above-mentioned flask was placed in a ice bath, while 10 mL of 95% by mass of formic acid was added dropwise under gradual stirring from the dropping funnel in the flask over a period of 2 hours. After the dropwise adding, the flask was taken out from the ice bath, the resultant mixture therein was further reacted for approximately 2 hours at room temperature. Thereafter mixed reaction liquid thus formed was transferred in one liter of crushed ice, and the resultant white crystal was filtered with a glass filter. In addition, the white crystal was dissolved in 50 mL of 30% by mass of aqueous solution of sodium hydroxide, and was filtered with a glass filter. To the resultant filtrate was added 100 mL of 95% by mass of sulfuric acid, so that white crystal was precipitated. The precipitated white crystal was filtered, washed with water and further washed with methanol and thus 1,3-adamantanedicarboxylic acid was obtained as described hereunder. yield amount: 24.365 g (108.65 millimol), yield rate: 85.0%, purity: 92.8%
References:
EP1398309,2004,A1 Location in patent:Page 5
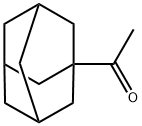
1660-04-4
259 suppliers
$6.00/1g

39269-10-8
180 suppliers
$9.00/1g
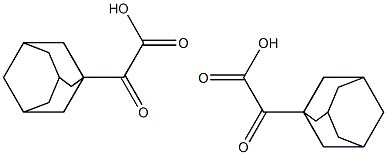
16091-98-8
3 suppliers
inquiry

39269-10-8
180 suppliers
$9.00/1g
![Ethanone, 2-hydroxy-1-tricyclo[3.3.1.13,7]dec-1-yl-](/CAS/20210305/GIF/33705-31-6.gif)
33705-31-6
0 suppliers
inquiry

39269-10-8
180 suppliers
$9.00/1g