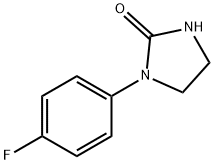
1-(4-Fluorophenyl)tetrahydro-2H-imidazol-2-one synthesis
- Product Name:1-(4-Fluorophenyl)tetrahydro-2H-imidazol-2-one
- CAS Number:53159-75-4
- Molecular formula:C9H9FN2O
- Molecular Weight:180.18
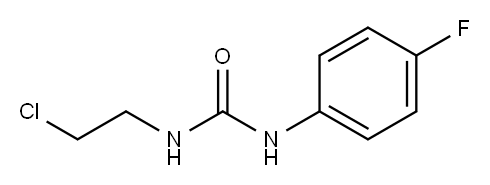
13908-32-2
24 suppliers
$59.00/1g

53159-75-4
25 suppliers
$55.85/250mgs:
Yield:53159-75-4 86%
Reaction Conditions:
with sodium hydride in tetrahydrofuran at 0 - 20; for 15 h;
Steps:
1-(4-Fluorophenyl)imidazolidin-2-one (2u)
To a solution of 2u-1 (0.5 g, 2.3 mmol) in THF (12 ml), 60 % NaH (0.1 g, 2.5 mmol) was added at 0oC and the mixture was stirred at room temperature for 15 hours at ambient temperature. The reaction mixture was quenched with sat. NH4Cl and extracted with EtOAc. The combined extracts were washed with brine, dried over MgSO4 and evaporated down. The residue was purified by silica gel column chromatography to give 0.36 g of desired 2u in 86 % yield.
References:
Teno, Naoki;Gohda, Keigo;Wanaka, Keiko;Tsuda, Yuko;Sueda, Takuya;Yamashita, Yukiko;Otsubo, Tadamune [Bioorganic and Medicinal Chemistry,2014,vol. 22,# 7,p. 2339 - 2352] Location in patent:supporting information
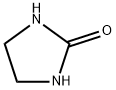
120-93-4
534 suppliers
$10.00/5g
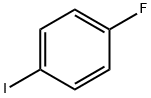
352-34-1
369 suppliers
$7.00/5g

53159-75-4
25 suppliers
$55.85/250mgs:
371-40-4
402 suppliers
$5.00/5G

53159-75-4
25 suppliers
$55.85/250mgs: