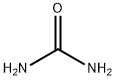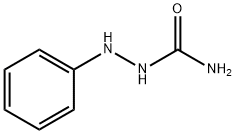
1-PHENYLSEMICARBAZIDE synthesis
- Product Name:1-PHENYLSEMICARBAZIDE
- CAS Number:103-03-7
- Molecular formula:C7H9N3O
- Molecular Weight:151.17
Yield:-
Reaction Conditions:
with hydrazine hydrate in tetrahydrofuran
Steps:
4.7 (7)
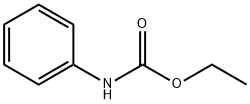
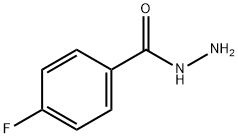
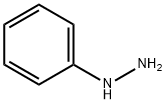
The solvent is then evaporated and the residue purified by liquid-gel partition chromatography using a 400*15 mm column of Sephadex LH20 substituted with Nedox 1114 olefin oxide to 20% w/w and eluding with dichloroethane/hexane/ethanol (100:100:5 v/v/v) containing 0.1% v/v of acetic acid. The chromatography gives the title compound as an oil (87 mg), δ(CDCl3) 9.60 (br, 1H), 8.10 (br, 1H), 7.7-7.1 (m, 5H), 5.35 (m, 2H). The phenyl semicarbazide is prepared as follows. Ethyl-N-phenyl carbamate (8.25 g) is refluxed with hydrazine hydrate (3.75 g) for 3 hours. The mixture is evaporated to dryness and the residue is treated with ether, and the solid phenyl semicarbazide (1.5 g) is filtered off, washed with ether and dessicated, m.p. 122°-124° C. Note: In a variant of the procedure described in this Example the acid/aldehyde (6) (100 mg) is reacted with p-fluorobenzoic hydrazide STR24 (45 mg) in place of the phenyl semicarbazide STR25 the reaction being carried out in tetrahydrofuran for 1.5 hours at 40° C. to yield the alternative bicyclo[2,2,2]octane derivative, trans-5-(6'-carboxyhex-2'Z-enyl)-6-[N-(p-fluorobenzoyl)-hydrazono methyl]-bicyclo[2,2,2]octane.
References:
National Research Development Corporation US4596823, 1986, A

5877-04-3
1 suppliers
inquiry

103-03-7
110 suppliers
$40.00/25g