
2-Propanesulfinamide, N-[1-(2-fluoro-5-nitrophenyl)ethylidene]-2-methyl-, [N(E),S(R)]- synthesis
- Product Name:2-Propanesulfinamide, N-[1-(2-fluoro-5-nitrophenyl)ethylidene]-2-methyl-, [N(E),S(R)]-
- CAS Number:1075230-62-4
- Molecular formula:C12H15FN2O3S
- Molecular Weight:286.32
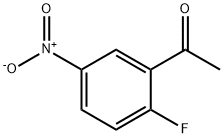
79110-05-7
167 suppliers
$20.00/5g
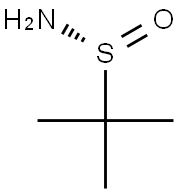
196929-78-9
376 suppliers
$4.00/1g
![2-Propanesulfinamide, N-[1-(2-fluoro-5-nitrophenyl)ethylidene]-2-methyl-, [N(E),S(R)]-](/CAS/20211123/GIF/1075230-62-4.gif)
1075230-62-4
7 suppliers
inquiry
Yield:1075230-62-4 91%
Reaction Conditions:
with titanium(IV) tetraethanolate in tetrahydrofuran at 70; for 12 h;Inert atmosphere;
Steps:
1.1 Step 1) Synthesis of (R,E)-N-(1-(2-fluoro-5-nitrophenyl)ethylidene)-2-methylpropane-2-sulfinamide
1-(2-Fluoro-5-nitro-phenyl)ethanone (5 g, 27.3 mmol), (R)-(+)-tert-butylsulfinamide (3.8 g, 31 mmol) and anhydrous tetrahydrofuran ( 25 mL) was placed in a 100 mL one-neck round bottom flask, and tetraethyl titanate (13.7 g, 60.1 mmol) was added, and the reaction was carried out at 70 ° C for 12 hours under nitrogen atmosphere. After the reaction was completed, the mixture was cooled to room temperature. The reaction mixture was poured into water (50 mL), filtered, and the filter cake was washed with dichloromethane (40 mL×2). /ethyl acetate (v/v)=20/1~5/1) get the titleThe compound was a yellow solid (7.1 g, 91.0%).
References:
CN108997328,2018,A Location in patent:Paragraph 0202; 0204; 0205

79110-05-7
167 suppliers
$20.00/5g
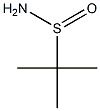
146374-27-8
243 suppliers
$9.00/5g
![2-Propanesulfinamide, N-[1-(2-fluoro-5-nitrophenyl)ethylidene]-2-methyl-, [N(E),S(R)]-](/CAS/20211123/GIF/1075230-62-4.gif)
1075230-62-4
7 suppliers
inquiry
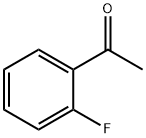
445-27-2
442 suppliers
$9.00/25g
![2-Propanesulfinamide, N-[1-(2-fluoro-5-nitrophenyl)ethylidene]-2-methyl-, [N(E),S(R)]-](/CAS/20211123/GIF/1075230-62-4.gif)
1075230-62-4
7 suppliers
inquiry