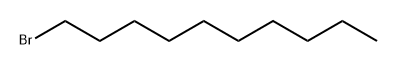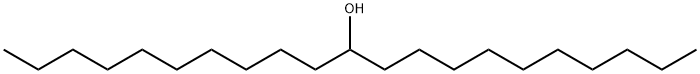
11-HENEICOSANOL synthesis
- Product Name:11-HENEICOSANOL
- CAS Number:3381-26-8
- Molecular formula:C21H44O
- Molecular Weight:312.57
Yield: 46%
Reaction Conditions:
Stage #1: with iodine;magnesiumBunsen flame;
Stage #2:1-bromo dodecane in tetrahydrofuran at 40; for 2 h;
Stage #3:undecylaldehyde with water;ammonium chloridemore than 3 stages;
Steps:
8
Magnesium turnings (705 mg, 28.9 mmol) and iodine (10 mg, catalytic) were combined and heated over a Bunsen flame under N2 until I2 gas evolved. The flask was allowed to cool, and then dry THF (100 mL) was added. Bromodecane (5.0 mL, 24.1 mmol) was added and the mixture was stirred for 2 h at 40 °C under N2. After this time, undecylic aldehyde (5.0 mL, 24.1 mmol) was added and the reaction was stirred for a further 1 h, at 55 °C, under N2. The reaction was quenched with sat. aq. NH4C1, and the solvent was evaporated under reduced pressure. The residue was diluted with CH2C12 (300 mL) and extracted with aq. NaCl (200 mL) followed by water (200 mL). The organic layer was dried over Na2SO4, filtered, and solvent removed under reduced pressure. The residue was bonded to silica (dissolved in EtOAc and evaporated in the presence of silica) and chromatographed (hexane-DCM 2: 1. TLC; Rf 0.57, Hex-EtOAc 6: 1) to furnish 11-heneicosanol as a white powder (3.44 g, 46%). 1H NMR (300 MHz, CDC13) : No. 3.58 (m, 1 H, OCHR2), 1.26-1. 48 (m, 36 H, 18 x CH2), 0.88 (app t, 6 H, J 6.9 Hz, 2 x CH3) ; 13C NMR (75.5 MHz, CDC13) : 8 72.0 (CH), 37.5, 31.9, 29.7, 29.6, 29.3, 25.7, 22.7 (18 x CH2), 14.1 (2 x CH3) ; LRMS (ESI) m/z 335 [ (M + Na) + 8%] 413 (100) 489 (11).
References:
GRIFFITH UNIVERSITY WO2003/97657, 2003, A1 Location in patent:Page/Page column 27

1002-69-3
220 suppliers
$7.00/25g
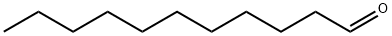
112-44-7
217 suppliers
$15.00/25g

3381-26-8
41 suppliers
$56.89/1g
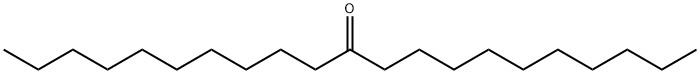
19781-72-7
52 suppliers
$90.00/1g

3381-26-8
41 suppliers
$56.89/1g
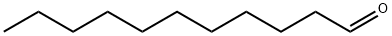
112-44-7
217 suppliers
$15.00/25g
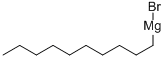
17049-50-2
48 suppliers
$59.50/100ml

3381-26-8
41 suppliers
$56.89/1g