
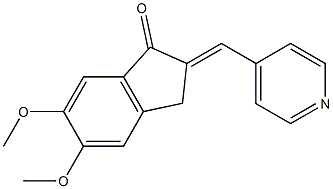
5,6-Dimethoxy-2-(piperidin-4-yl)methylene-indan-1-one synthesis
- Product Name:5,6-Dimethoxy-2-(piperidin-4-yl)methylene-indan-1-one
- CAS Number:120014-30-4
- Molecular formula:C17H23NO3
- Molecular Weight:289.37
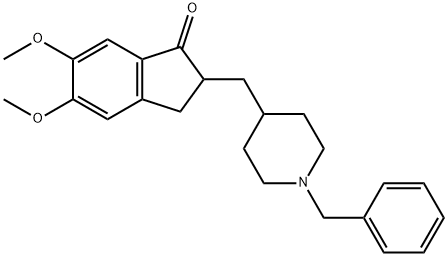
120014-06-4
331 suppliers
$5.00/100mg

120014-30-4
148 suppliers
$300.00/100g
Yield:120014-30-4 97%
Reaction Conditions:
with ammonium formate;palladium 10% on activated carbon in methanol at 20; for 3 h;Heating / reflux;
Steps:
1; 4 Synthesis of 5,6-dimethoxy-2-(piperidin-4-ylmethyl)-2,3-dihydro-1H-inden-1-one 12 (Scheme 4)
Example 1 Synthesis of 5,6-dimethoxy-2-(piperidin-4-ylmethyl)-2,3-dihydro-1H-inden-1-one 12 (Scheme 4) To a stirred suspension of palladium, 10 wt. % on activated carbon (4 g) in methanol (25 ml) at room temperature under nitrogen atmosphere was added a solution of indanone 11 (0.01 mol, 4.1 g) in methanol (25 ml) followed by ammonium formate (0.07 mol, 4.40 g). The resulting mixture was refluxed for 3 hours. The progress of the reaction was monitored by TLC. The reaction mixture was cooled to room temperature and filtered through a Celite pad. The filtrate was concentrated on rotavapor and the residue was diluted with DCM (150 ml). The DCM solution was washed with water, dried over anhydrous sodium sulfate (Na2SO4) and evaporated the solvent. The residue was triturated with hexane to give the target indanone 12 as white solid in 97% yield (2.80 g). 1H NMR (400 MHz, CDCl3): δ 1.16-0.92 (3H, m); 1.58-1.40 (3H, m); 1.70-1.64 (1H, m); 2.51-2.39 (4H, m); 2.77 (1H, broad s); 2.92-2.87 (2H, m); 3.05-2.09 (1H, m); 3.67 (3H, s); 3.74 (3H, s); 6.66 (1H, s); 6.92 (1H, s). MS (ESI): m/z=290.20 (M+H+).
References:
Reviva Pharmaceuticals, Inc. US2008/153878, 2008, A1 Location in patent:Page/Page column 13; 20
4803-57-0
83 suppliers
$70.00/25mg

120014-30-4
148 suppliers
$300.00/100g
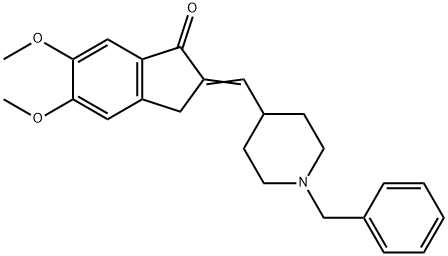
120014-07-5
137 suppliers
$16.00/250mg

120014-06-4
331 suppliers
$5.00/100mg

120014-30-4
148 suppliers
$300.00/100g
948550-60-5
10 suppliers
$174.90/5 mg

120014-30-4
148 suppliers
$300.00/100g
877606-65-0
3 suppliers
inquiry

4803-57-0
83 suppliers
$70.00/25mg

120014-30-4
148 suppliers
$300.00/100g