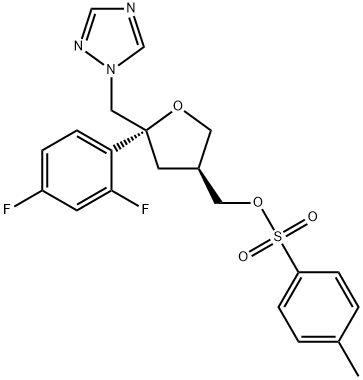
(5R-cis)-Toluene-4-sulfonic acid 5-(2,4-difluorophenyl)-5-(1H-1,2,4-triazol-1-yl)methyltetrahydrofuran-3-ylmethyl ester synthesis
- Product Name:(5R-cis)-Toluene-4-sulfonic acid 5-(2,4-difluorophenyl)-5-(1H-1,2,4-triazol-1-yl)methyltetrahydrofuran-3-ylmethyl ester
- CAS Number:149809-43-8
- Molecular formula:C21H21F2N3O4S
- Molecular Weight:449.47

98-59-9
586 suppliers
$9.00/5g
160709-02-4
63 suppliers
inquiry

149809-43-8
371 suppliers
$56.00/100mg
Yield:149809-43-8 215 g
Reaction Conditions:
with dmap in dichloromethane at 0 - 30; for 8.25 h;Reagent/catalyst;Solvent;
Steps:
12 Example-12: Preparation of ((3S,5R)-5-((1H-i,2,4-triazol-1-yl)methyl)-5-(2,4-difluoro phenyl) tetrahydrofuran-3-yl)methyl 4-methylbenzenesulfonate (Formula-i)
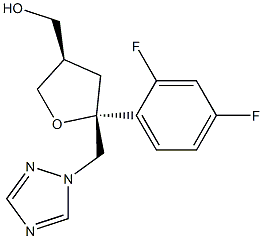
Example-12: Preparation of ((3S,5R)-5-((1H-i,2,4-triazol-1-yl)methyl)-5-(2,4-difluoro phenyl) tetrahydrofuran-3-yl)methyl 4-methylbenzenesulfonate (Formula-i)Dimethyl aminopyridine (114 gins), followed by para toluene sulfonyl chloride (161 gins) were slowly added to a pre-cooled solution of dichioromethane (1250 ml) and ((3R,5R)-5-((1 H-i ,2,4-triazol- 1 -yl)methyl)-5-(2,4-difluorophenyl) tetrahydrofuran-3-yl)methanol (250 gins) at 0-5°C. Raised the temperature of the reaction mixture to 25-30°C and stirred for 8 hours at the same temperature. Water was added to the reaction mixture at 25-30°C and stirred for 15 minutes at the same temperature. Both the organic and aqueous layers were separated and washed the organic layer with 10% aqueous hydrochloric acid solution, followed by 10% aqueous sodiumcarbonate solution. Organic layer is washed with water. Distilled off the solvent from the organic layer and co-distilled with petroleum ether. Ethanol (1.00 ml) were added to the obtained solid at 25-30°C and heated to 60-65°C and then cooled to 25-30°C and stirred for 8 hrs at the same temperature. Further, cooled to 0-5 and filtered the precipitated solid, washed with ethanol and then dried to get the title compound. Yield: 215 gms. Purity by HPLC: 99.98%.
References:
MSN LABORATORIES PRIVATE LIMITED;THIRUMALAI RAJAN, Srinivasan;ESWARAIAH, Sajja;SAHADEVA REDDY, Maramreddy WO2015/59716, 2015, A2 Location in patent:Page/Page column 22
1350466-82-8
0 suppliers
inquiry

98-59-9
586 suppliers
$9.00/5g

149809-43-8
371 suppliers
$56.00/100mg

182210-71-5
17 suppliers
inquiry

149809-43-8
371 suppliers
$56.00/100mg
182009-37-6
0 suppliers
inquiry

149809-43-8
371 suppliers
$56.00/100mg
165115-78-6
9 suppliers
inquiry

149809-43-8
371 suppliers
$56.00/100mg