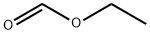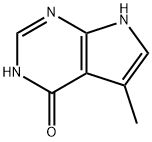
4H-Pyrrolo[2,3-d]pyrimidin-4-one, 1,7-dihydro-5-methyl- (9CI) synthesis
- Product Name:4H-Pyrrolo[2,3-d]pyrimidin-4-one, 1,7-dihydro-5-methyl- (9CI)
- CAS Number:1618-37-7
- Molecular formula:C7H7N3O
- Molecular Weight:149.15
Yield:1618-37-7 8.65 g
Reaction Conditions:
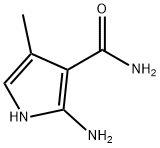
Stage #1: 2-amino-4-methyl-1H-pyrrole-3-carboxamidewith sodium methylate in methanol at 55; for 3 h;
Stage #2: formic acid ethyl ester in methanol at 55;
Steps:
5-Methyl-7H-pyrrolo[2,3-d]pyrimidin-4-ol (1)
To a solution of LiOH (3.5 g, 147.7 mmol, 1.5 equiv.) in anhydrous MeOH (120 mL) was added cyanoacetamide (12.4 g, 147.7 mmol, 1.5 equiv.) under argon. This solution was stirred 20 min and addition of a solution of phtalimidoacetone in anhydrous THF (140 mL) was performed over 30 min. The reaction mixture was then stirred 2 h at room temperature and 1 h at 55°C. A sodium methoxide (NaOMe) solution (25% in methanol, 31.6 mL, 147.7 mmol, 1.5 equiv.) was added dropwise at 55°C and the mixture was stirred 3 h. Ethyl formate (HCOOEt, 39.7 mL, 492.2 mmol, 5.0 equiv.) followed by NaOMe solution (25% in methanol, 63.2 mL, 295.3 mmol, 3.0 equiv.) were then added and the reaction mixture was stirred at 55°C overnight. After cooling, the reaction mixture was diluted with water (500 mL), heated at 60°C for 1 h, and then concentrated under reduced pressure to small volume (≈ 500 mL). The resulting solution was slowly acidified to pH = 7 with a 6 N aqueous HCl solution, cooled to about 5°C and stirred at this temperature for 30 min. The solid was filtered, washed with water (100 mL) and dried at 50°C under vacuum overnight to afford compound 1 (8.65 g, 59%) as a light brown solid. 1H NMR (250 MHz, DMSO-d6) δ 11.50 (br s, 2H, 2 x NH), 7.73 (s, 1H, CH), 6.75 - 6.72 (m, 1H, CH), 2.26 (d, J = 1.1 Hz, 3H, CH3). Org. Process Res. Dev. 2007, 11 (1), 86-89.
References:
EP3915990,2021,A1 Location in patent:Paragraph 0106-0107

1020110-76-2
0 suppliers
inquiry
![4H-Pyrrolo[2,3-d]pyrimidin-4-one, 1,7-dihydro-5-methyl- (9CI)](/CAS/GIF/1618-37-7.gif)
1618-37-7
23 suppliers
inquiry