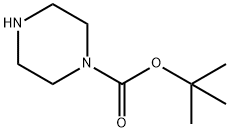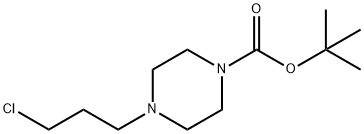
4-(3-CHLORO-PROPYL)-PIPERAZINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER synthesis
- Product Name:4-(3-CHLORO-PROPYL)-PIPERAZINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER
- CAS Number:165530-45-0
- Molecular formula:C12H23ClN2O2
- Molecular Weight:262.78
Yield: 82%
Reaction Conditions:
with potassium carbonate;sodium iodide in acetonitrile at 20;
Steps:
2.2-5.2 Step 2: Preparation of Compound II-5c
Compound II-5b (20.24 g, 101.06 mmol) was dissolved in acetonitrile (200 mL) and 1-bromo-3-chloropropane (39.77 g, 252.65 mmol, 2.5 eq), potassium carbonate (13.97 g, 110.78 mmol) Sodium iodide (15.15 g, 101.06 mmol) was added and stirred overnight at room temperature. After the reaction was completed, the reaction solvent was concentrated under reduced pressure, ethyl acetate, distilled water and brine were sequentially washed, and the organic layer was dried over anhydrous magnesium sulfate and concentrated under reduced pressure. The concentrated residue was purified by silica gel column chromatography (dichloromethane: methanol = 20: 1-> 15: 1 volume ratio mixture gradient elution) to give the title compound II-5c (22.93 g, 82%).
References:
Selteurion Co., Ltd.;Park Yeong-jun;Song Hyeon-nam;Park Seong-jun;Jeong Jin-gyo;Ryu Hyeon;Cha Ji-hyeon;Choi Jun-heon;Seo Won-il;Kim Min-cheol;Park So-jin;Hyun Jae-gyeong KR2019/143246, 2019, A Location in patent:Paragraph 0369; 0491; 0498-0502

24424-99-5
817 suppliers
$13.50/25G

165530-45-0
29 suppliers
inquiry
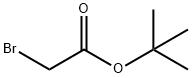
5292-43-3
442 suppliers
$10.00/5g

165530-45-0
29 suppliers
inquiry