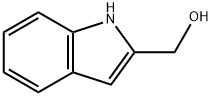
1H-INDOL-2-YLMETHANOL synthesis
- Product Name:1H-INDOL-2-YLMETHANOL
- CAS Number:24621-70-3
- Molecular formula:C9H9NO
- Molecular Weight:147.17
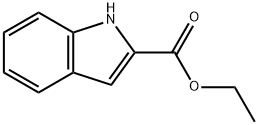
3770-50-1
319 suppliers
$10.00/5g

24621-70-3
129 suppliers
$40.00/1 g
Yield:24621-70-3 100%
Reaction Conditions:
Stage #1:2-carbethoxyindole with lithium aluminium tetrahydride in tetrahydrofuran at 0;
Stage #2: with water;sodium hydroxide in tetrahydrofuran
Steps:
6
Compound 17: (1H-indol-2-yl)methanolTo ethyl 1H-indole-2-carboxylate 16 (6.5 g, 34.35 mmol) in THF at 0° C. was added lithium aluminum hydride solution (1M, in THF 1.43 g, 37.78 mmol) dropwise and the reaction mixture was stirred for 3.5 hours at 0° C. The reaction mixture was quenched with H2O, 15% NaOH, and H2O before it was filtered and rinsed with THF. Reaction mixture was dried (anhydrous Na2SO4) and evaporation of the solvent gave 5.37 g (100% yield) of the crude (1H-indol-2-yl)methanol 17 which was used directly in the next step.
References:
Neosome Life Sciences, LLC US2012/302578, 2012, A1 Location in patent:Page/Page column 23
1477-50-5
493 suppliers
$6.00/5g

24621-70-3
129 suppliers
$40.00/1 g
1202-04-6
217 suppliers
$6.00/1g

24621-70-3
129 suppliers
$40.00/1 g
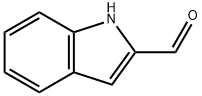
19005-93-7
196 suppliers
$7.00/100mg

24621-70-3
129 suppliers
$40.00/1 g
![Pyrazino[1,2-a:4,5-a]diindole-6,13-dione](/CAS/20180906/GIF/58881-41-7.gif)
58881-41-7
0 suppliers
inquiry

19005-93-7
196 suppliers
$7.00/100mg

24621-70-3
129 suppliers
$40.00/1 g